4850
Intrinsic susceptibility MRI can inform on tumour vascular decompression in response to the stromal targeted therapy PEGPH20
Emma L. Reeves1, Jin Li1, Jessica K. R. Boult1, Barbara Blouw2, David Kang2, Jeffrey C. Bamber1, Yann Jamin1, and Simon P. Robinson1
1Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 2Halozyme Therapeutics, San Diego, CA, United States
1Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 2Halozyme Therapeutics, San Diego, CA, United States
Synopsis
PEGPH20 has been shown to decompress blood vessels and improve tumour drug delivery. Given the sensitivity of R2* to paramagnetic deoxyhaemoglobin and reflection of RBC content in tumour vasculature, we hypothesised that IS-MRI may provide a useful biomarker of PEGPH20 response. We performed IS-MRI before and after PEGPH20 treatment in 4T1 HAS3 and MDA-MB-231 LM2-4 orthotopic breast tumours. R2* significantly increased following PEGPH20 treatment in 4T1 HAS3 tumours, however, R2* was unable to detect a similar response to PEGPH20 in the relatively hypovascular MDA-MB-231 LM2-4 tumours. Another vascular biomarker may provide greater sensitivity and detect PEGPH20 response in hypovascular tumours.
Introduction
The dense fibrotic stroma typically associated with breast and pancreatic cancer compresses tumour blood vessels, thereby impairing efficient drug delivery. Significant efforts are focussed on targeting the tumour extracellular matrix (ECM) for therapeutic gain. Degradation of hyaluronan (HA), a major constituent of the ECM, by PEGPH20 (a PEGylated, recombinant human hyaluronidase) has been shown pre-clinically to decompress tumour blood vessels and improve drug delivery1-4. Non-invasive monitoring of this process may identify appropriate timepoints for optimal drug delivery.The transverse relaxation rate (R2*), quantified using intrinsic susceptibility (IS)-MRI, is sensitive to the concentration of paramagnetic deoxyhaemoglobin within tissue vasculature. Tumours often exhibit relatively fast R2* compared to most normal tissues, a consequence of the high concentration of deoxygenated red blood cells (RBCs) within the typically chaotic and unstable microcirculation. IS-MRI has thus been utilised to investigate tumour angiogenesis and response to vascular targeted therapies5,6. We recently showed that parametric maps of tumour R2* reflect the spatial variation in RBCs seen histologically6. Given that blood vessel decompression is likely to increase the number of RBCs in the vasculature, we hypothesised that R2* may provide a sensitive endogenous imaging biomarker of breast tumour response to PEGPH20.
Methods
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Murine 4T1 breast tumours which overexpress HA synthase (HAS3) and human luc-MDA-MB-231 LM2-4 breast tumours were propagated orthotopically in female BALB/c mice (n = 12) and athymic NCr-Foxn1nu mice (n = 12) respectively. 4T1 HAS3 (362 ± 33 mm3) and MDA-MB-231 LM2-4 (456 ± 38 mm3) tumours were imaged prior to and 24 hours after treatment with either saline or 1 mg/kg PEGPH20 (Halozyme Therapeutics).MRI was performed using a 7T Biospec 70/20 USR horizontal MRI system (Bruker Instruments, Ettlingen, Germany). Tumour R2* was quantified using a multiple gradient echo (MGE) sequence (TE = 3 ms, TESPACE = 3 ms, 8 echoes, TR = 200 ms, α = 45°). Parametric maps of R2* were constructed from a 1 mm thick axial slice over a 3x3 cm FOV taken from the tumour centre. Median R2* values were calculated from a region of interest which contained all viable tumour tissue and excluded necrosis. MRI-aligned serial tissue sections (5 µm) were cut and HA was detected by a biotinylated, recombinant HA-binding protein7 (HTI-601; Halozyme Therapeutics). Immunohistochemical staining of CD31 was used to evaluate tumour microvasculature, and was quantified using Definiens Tissue Studio®.
Statistical analyses were performed using Prism 8 (GraphPad) and data are presented as mean ± SEM. Significant differences were identified using a Mann-Whitney U test with a significance level of 5%.
Results
PEGPH20 treatment induced a cytolentic response in both 4T1 HAS3 and MDA-MB-231 LM2-4 tumours over 24 hours. R2* significantly increased in 4T1 HAS3 tumours following PEGPH20 treatment (Figure 1A & B). R2* increased from 62 ± 2 s-1 to 82 ± 7 s-1 in the PEGPH20 treated 4T1 HAS3 tumours whilst R2* decreased from 71 ± 3 s-1 to 65 ± 5 s-1 in saline control 4T1 HAS3 tumours. PEGPH20 treatment did not significantly change R2* in MDA-MB-231 LM2-4 tumours (Figure 2A & B). R2* of PEGPH20 treated MDA-MB-231 LM2-4 tumours increased from 40 ± 2 s-1 to 52 ± 5 s-1, with a similar increase seen in the saline control MDA-MB-231 LM2-4 tumours from 55 ± 5 s-1to 64 ± 8 s-1. The within-subject test-retest coefficient of variation for R2* was 19% when using pre- and post-saline data for both tumour models.HTI-601 staining showed that 4T1 HAS3 tumours exhibited more HA accumulation than MDA-MB-231 LM2-4 tumours, and that PEGPH20 treatment successfully degraded HA in both breast tumour models (Figure 3A). CD31 staining highlighted that 4T1 HAS3 tumours had a significantly higher vessel density than MDA-MB-231 LM2-4 tumours (Figure 3A & B).
Discussion
4T1 HAS3 tumours exhibited a faster baseline R2* than MDA-MB-231 LM2-4 tumours, suggesting that 4T1 HAS3 tumours have more haemodynamic vasculature; this was corroborated by the CD31 staining. The increase in R2* following PEGPH20 treatment in 4T1 HAS3 tumours is consistent with decompression of blood vessels and increased accessibility of RBCs within the tumour microenvironment. The lack of change in R2* after PEGPH20 treatment in MDA-MB-231 LM2-4 tumours may suggest that vessel decompression is not always correlated with an increase in RBCs, or that decompression of a small number of blood vessels does not create a large enough change in magnetisation to be detected by R2*. These data suggest that R2* is a sensitive biomarker of PEGPH20 response in well vascularised tumours, such as 4T1 HAS3. However, alternative vascular biomarkers may provide greater specificity to PEGPH20 response in hypovascular tumours. For example, the volume transfer constant (Ktrans), quantitated from DCE-MRI, has been shown to increase following PEGPH20 treatment in pre-clinical and human pancreatic ductal adenocarcinomas (PDAC)8,9. We plan to investigate the utility of susceptibility contrast MRI with ultrasmall superparamagnetic iron oxide (USPIO) particles to quantify tumour blood volume response to PEGPH20.Conclusion
Quantitation of R2* by IS-MRI can detect blood vessel decompression following PEGPH20 treatment in well vascularised 4T1 HAS3 breast tumours. However, R2* was unable to detect a similar response in relatively hypovascular MDA-MB-231 LM2-4 tumours.Acknowledgements
We acknowledge funding from the Cancer Research UK Centre at the ICR, and from the Cancer Research UK Imaging Centre at the ICR, funding from the Cancer Research UK grant C16412/A27725, and European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039, in association with a Children with Cancer UK Research Fellowship.References
- Provenzano, P.P., et al., Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma.Cancer Cell, 2012. 21(3): p. 418-29.
- Clift, R., et al., Remodeling the Tumor Microenvironment Sensitizes Breast Tumors to Anti-Programmed Death-Ligand 1 Immunotherapy.Cancer Research, 2019. 79(16): p. 4149.
- Thompson, C.B., et al., Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models.Mol Cancer Ther, 2010. 9(11): p. 3052-64.
- DuFort, Christopher C., et al., Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase.Biophysical Journal, 2016. 110(9): p. 2106-2119.
- Jamin, Y., et al., Intrinsic susceptibility MRI identifies tumors with ALKF1174L mutation in genetically-engineered murine models of high-risk neuroblastoma.PloS one, 2014. 9(3): p. e92886-e92886.
- Zormpas-Petridis, K., et al., MRI Imaging of the Hemodynamic Vasculature of Neuroblastoma Predicts Response to Anti-angiogenic Treatment.Cancer Research, 2019.
- Jadin, L., et al., Characterization of a Novel Recombinant Hyaluronan Binding Protein for Tissue Hyaluronan Detection.Journal of Histochemistry & Cytochemistry, 2014. 62(9): p. 672-683.
- Cao, J., et al., Dynamic contrast-enhanced MRI detects responses to stroma-directed therapy in mouse models of pancreatic ductal adenocarcinoma.Clin Cancer Res, 2018.
- Hingorani, S.R., et al., Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer.Clin Cancer Res, 2016. 22(12): p. 2848-54.
Figures

Figure 1. (A) Anatomical T2-weighted MRI and parameter maps of R2* obtained from two 4T1 HAS3 orthotopic breast tumours prior to and 24 hours after treatment with either saline or PEGPH20 (1 mg/kg). (B) Percentage change in R2* between pre- and post-treatment MRI for all saline (n = 6) and PEGPH20 (n = 6) treated 4T1 HAS3 tumours. PEGPH20 significantly increased R2* in 4T1 HAS3 tumours (p = 0.008).

Figure 2. (A) Anatomical T2-weighted MRI and parameter maps of R2* obtained from two MDA-MB-231 LM2-4 orthotopic breast tumours prior to and 24 hours after treatment with either saline or PEGPH20 (1 mg/kg). Note the decrease in R2* scale compared to Figure 1. (B) Percentage change in R2* between pre- and post-treatment MRI for all saline (n = 6) and PEGPH20 (n = 6) treated MDA-MB-231 LM2-4 tumours. PEGPH20 treatment did not significantly change R2* in MDA-MB-231 LM2-4 tumours (p = 0.937).
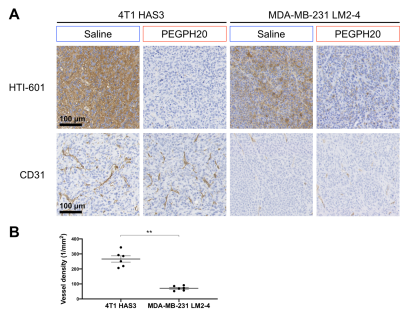
Figure 3. (A) Representative images (20x) of 4T1 HAS3 and MDA-MB-231 LM2-4 tumours stained with HTI-601 or CD31. One saline control and one PEGPH20 treated tumour are shown per in vivo model. HTI-601 staining reveals a marked reduction in HA with PEGPH20 treatment. (B) Vessel density, quantified from CD31 staining, for all saline control 4T1 HAS3 (n = 6) and MDA-MB-231 LM2-4 (n = 6) tumours. 4T1 HAS3 tumours had significantly higher vessel density than MDA-MB-231 LM2-4 tumours (p = 0.002).