4846
Prediction of Treatment Outcome after Radiosurgery in Brain Metastases from Lung Cancer Using Preradiosurgical MR Radiomics1Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 2Department of Neurosurgery, Neurological Institute, Taipei Veteran General Hospital, Taipei, Taiwan, 3School of Medicine, National Yang-Ming University, Taipei, Taiwan, 4Brain Research Center, National Yang-Ming University, Taipei, Taiwan, 5Department of Radiology, Taipei Veteran General Hospital, Taipei, Taiwan, 6Department of Nuclear Medicine and National PET/Cyclotron Center, Taipei Veterans General Hospital, Taipei, Taiwan, 7Institute of Biophotonics, National Yang-Ming University, Taipei, Taiwan
Synopsis
The development of brain metastases is a devastating consequence of disease progression of advanced non-small cell lung cancer patients. This study proposed an approach to predict the treatment outcome after gamma knife radiosurgery based on preradiosurgical MR radiomics. We suggested that imaging characteristics extracted from preradiosurgical MRIs can be potential image biomarkers for the outcome prediction.
Background and Purpose
About 35% of non-small cell lung cancer (NSCLC) patients developed BM during the disease course, and 15 to 25% of advanced NSCLC patients had BM at initial diagnosis.1 In past decades, Gamma Knife radiosurgery (GKRS) has become one of the first-line treatment for BM.2, 3 Several recent studies further reported that EGFR mutation was associated with favorable outcomes in NSCLC-BM patients treated with GKRS.4-6 In this study, we aim to investigate whether the imaging characteristics extracted from preradiosurgical MRIs can predict the treatment outcome following GKRS, including tumor control of BM and overall survival, and reflect the EGFR mutation.Materials and Methods
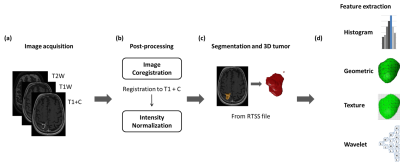
Clinical data and MRIs from 240 NSCLC-BM patients who received GKRS treatment were retrospectively collected in this study. Inclusion criteria were as follows: 1) diagnosis of NSCLC confirmed by lung biopsy or surgery with available EGFR mutation status; 2) diagnosis of one or several brain metastases confirmed by MRI; 3) available clinical and neuroimaging follow-up after GKRS at least once. EGFR mutations were found in 66.7% of the patients. The clinical characteristics of recruited patients are listed in Table 1.Routine MRIs were acquired from 240 patients, including contrast-enhanced T1-weighted (T1+C), T1-weighted (T1W), and T2-weighted (T2W) images. Several processing steps on the MRIs were applied to improve the reliability of radiomics analysis. The adjustment of image resolution was first performed to resample all voxel size to 0.50 x 0.50 x 3.00 mm3 for each MR modalities. The T2W and T1W images were then registered to T1+C images using rigid body transformation followed by the image intensity normalization. The BM volume of interest was delineated based on treatment plan of GKRS. For patients with multiple BMs, only the largest BM was used for the radiomics analysis. Finally, 1763 3D-Radiomic features, including histogram, shape and size, and texture analyses, were extracted from all MRIs. The diagram of image processing is displayed in Fig. 1.
A two-step feature selection was applied to identify key radiomic features and reduce feature redundancy which can potentially improve the model efficacy. The first step was conducted by a two-sample t-test to identify the feature candidates with significant differences (p < 0.05) between groups. Final key features for training classification models were further sieved out by using the sequential forward selection algorithm. Support vector machine (SVM) classifiers were used, and the 30% hold-out validation method was applied for each classification.
Results
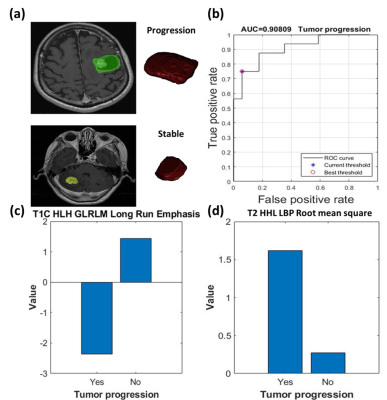
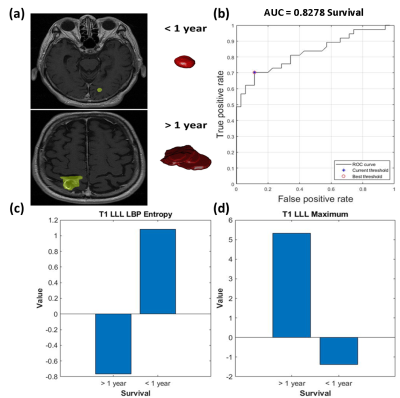
For the prediction of tumor control, 44 features were significantly different between patients with tumor progression and non-progression groups. The SVM classification was based on five features, including skewness in T1+C, long run emphasis in T1+C, mean of local binary pattern (LBP) in T2W, root mean square of LBP in T2W, and standard deviation of LBP in T2W, and achieved a sensitivity of 75%, a specificity of 93%, an accuracy of 85%, and an area under the curve (AUC) of receiver operating characteristic curve of 0.91 (Fig.2).For the prediction of overall survival, 74 features were significantly different between patients with longer survival (> 1 year) and poorer survival (< 1 year). The SVM classification was based on seven features (Including maximum in T1W and filtered T1W, entropy of LBP in T1W, autocorrelation in T1W, variance in T1W, Long Run Low Gray-Level Emphasis in T1+C, and first quartile in T2W) with a sensitivity of 70%, a specificity of 89%, an accuracy of 79%, and an AUC of 0.83 (Fig.3).
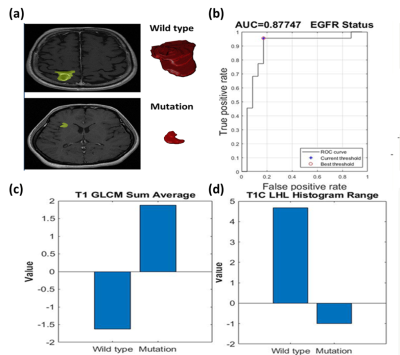
For the prediction of EGFR status, 30 features were significantly different between patients with EGFR mutation and wild type. The SVM classification was based on two features (sum average in T1W and range in T1+C). This model yielded a sensitivity of 95%, a specificity of 83%, an accuracy of 89%, and an AUC of 0.88 (Fig.4).
Conclusion
In the present study, we confirmed the feasibility of using preradiosurgical MR radiomics to predict the treatment outcome of NSCLC-BM after GKRS. We suggested that specific MR radiomic features may be potential image biomarkers for the outcome prediction.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-010-058-MY2, MOST 106-2221-E-010-016-MY3, MOST 107-2634-F075-001, MOST 108-2321-B-010-012-MY2).References
1. Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84.
2. Sheehan J, Kondzioka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: outcomes and prognostic factors. Journal of neurosurgery. 2005;102(Suppl):247-254.
3. Serizawa T, Ono J, Iichi T, Matsuda S, Sato M, Odaki M, Hirai S, Osato K, Saeki N, Yamaura A. Gamma knife radiosurgery for metastatic brain tumors from lung cancer: a comparison between small cell and non-small cell carcinoma. Journal of neurosurgery. 2002;97(5 Suppl):484-488.
4. Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, Lynch TJ, Sequist LV. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro-oncology. 2010 Jul 13;12(11):1193-9.
5. Yang WC, Xiao F, Shih JY, Ho CC, Chen YF, Tseng HM, Chen KY, Liao WY, Yu CJ, Yang JC, Kuo SH. Epidermal growth factor receptor mutation predicts favorable outcomes in non-small cell lung cancer patients with brain metastases treated with stereotactic radiosurgery. Radiotherapy and Oncology. 2018 Feb 1;126(2):368-74.
6. Lee CC, Hsu SP, Lin CJ, Wu HM, Chen YW, Luo YH, Chiang CL, Hu YS, Chung WY, Shiau CY, Guo WY. Epidermal growth factor receptor mutations: association with favorable local tumor control following Gamma Knife radiosurgery in patients with non–small cell lung cancer and brain metastases. Journal of neurosurgery. 2019 Jun 21;1(aop):1-8.
Figures