4844
VERDICT and kurtosis modelling of diffusion MRI for early assessment of radiotherapy response in a model of human neuroendocrine tumour
Lukas Carl Lundholm1, Mikael Montelius1, Oscar Jalnefjord1, Emman Shubbar1, Eva Forssell-Aronsson1, and Maria Ljungberg1
1Department of Radiation Physics, Institute of Clinical Sciences, Göteborg, Sweden
1Department of Radiation Physics, Institute of Clinical Sciences, Göteborg, Sweden
Synopsis
Diffusion MRI methods to characterize tumour tissue could facilitate early assessment of radiotherapy response. In this study mouse models of human small intestine neuroendocrine tumours were irradiated externally, and post-treatment effects were studied over two weeks using VERDICT and kurtosis analysis of diffusion MRI measurements. Results showed multiple correlations between tumour treatment response and parameters estimated from the models, suggesting that a decrease of cell density and microstructural barriers early after treatment indicates a more substantial response. Both VERDICT and kurtosis analysis shows great potential for early assessment of treatment response to external radiation therapy in the studied tumour model.
Introduction
Non-invasive methods for tumour tissue characterization would facilitate prediction and early assessment of therapy response and thereby personalized treatment planning. Diffusion weighted MRI (dMRI) is a promising technique for this purpose and holds the potential to indirectly estimate microstructural parameters beyond the conventional resolution limits of MRI. Two suggested dMRI based models are the Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours (VERDICT)1 and kurtosis (DKI) models. VERDICT aims to separate the MR signal coming from different tissue compartments and utilizes multiple diffusion gradient timings and strengths to estimate microstructural parameters such as cell size, intracellular (IC), vascular (VASC) and extracellular extravascular (EES) volume fractions. DKI estimates the presence of microstructural barriers and compartments by modelling the non-gaussian behaviour of the diffusing water molecules. DKI also provides estimates of the kurtosis-corrected apparent diffusion coefficient.To our knowledge, studies using VERDICT and DKI for assessment of therapy response in small-intestine neuroendocrine tumours (SI-NET) have not been conducted. The aim of this study was to investigate if VERDICT and DKI analysis can be used for early and longitudinal assessment of external radiation therapy response in SI-NETs.
Subjects and methods
Balb/C nude mice (n=20) with subcutaneous SI-NET (GOT1) xenografts were irradiated externally with an absorbed dose of 8 Gy to the tumour on day 0, using a 6 MV photon beam (Varian Medical Systems). In vivo dMRI experiments were conducted on days -1, 2, 3 as well as one week (day 6 or 7) and two weeks (day 15) after the treatment using a 7T preclinical MR system (Bruker, Biospec), table 1. The kurtosis model was fitted using an in-house built MATLAB (MathWorks, Natick, USA) software to the subset of the dMRI experiments with TE=39 ms. The VERDICT model was fitted to the full set of dMRI experiments using the AMICO framework2 where the EES and IC compartments were described using the BallSphere model, and the VASC compartment was described using a model which separates the blood flow from the diffusion of the blood3. To make the model fit more robust some parameters of the VERDICT model were fixed. A grid search was used to find the fixed parameter combination which yielded the lowest fitting cost function for the entire data set (dIC=1x10-9 m2/s, dEES=1.5x10-9 m2/s, dVASC=1.75x10-9 m2/s, vdVASC=0.6x10-3 m/s).Tumour volumes were measured externally on days -4, -1, 0, 2, 3, 7, and 15 using callipers. The tumour treatment response (TTR) was calculated according to
$$TTR=-ln\left(\frac{V_{15}}{V'_{15}}\right)$$
where V15 is the measured tumour volume on day 15 and V'15 is the hypothetical non-treated tumour volume on day 15 assuming an exponential growth based on volume measurements prior to the treatment4. Linear correlations between TTR and estimated diffusion parameters were analysed using the Pearson correlation coefficient (r).
The Gothenburg Ethical Committee on Animal Research approved this study.
Results & discussion
Out of the parameters estimated by VERDICT, the IC and EES volume fractions showed the most prominent trends early after treatment where the IC volume fraction tended to decrease and the EES volume fraction tended to increase during the first week (Fig 1), possibly indicating a reduction in cell density. The same trends could also be qualitatively analysed and observed in parameter maps of the estimated parameters (Fig 3), where the heterogeneity of the tumour tissues was revealed as well.Trends were also observed for the parameters estimated with DKI where the kurtosis-corrected diffusion coefficient (Dk) tended to increase and the kurtosis (K) tended to decrease during the first week (Fig 2), suggesting a reduction in microscopical compartments and diffusion barriers. After day 7 the progression of both VERDICT and DKI parameters showed a more scattered result.
Multiple linear correlations were found between TTR and estimated diffusion parameters early after treatment (Fig 4). A relative increase of Dk (r = 0.60) and EES volume fraction (r = 0.57) from day -1 to day 3 correlated with a higher TTR. On the contrary, a relative decrease of K (r = -0.61) and IC volume fraction (r = -0.67) from day -1 to day 3 correlated with a higher TTR. These results suggest that early signs of reduced cell density and microscopical barriers after treatment may indicate a more substantial response. Additionally, a higher cell radius on day 3 correlated with a higher TTR (r = 0.54).
Conclusion
Our results show that VERDICT and kurtosis modelling of dMRI data holds great potential for early assessment of tumour response to external radiation therapy in the GOT1 tumour model.Acknowledgements
No acknowledgement found.References
- Panagiotaki et al., NMR in Biomed. 2014
- Bonet-Carne et al., NMR in Biomed. 2018
- Ahlgren et al., NMR in Biomed. 2016
- Mehrara et al., Br J Cancer. 2011
Figures
Table 1: Scan protocol used for the
diffusion weighted MR measurements. A b0-image was acquired for
every segment of diffusion gradient parameters to compensate for varying echo
times
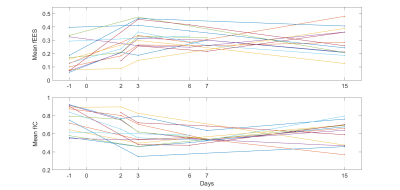
Figure 1: Mean values of extracellular extravascular
volume fraction (fEES, upper) and intracellular volume fraction (fIC, lower)
within the tumour as
estimated by VERDICT, for all included time points and mice
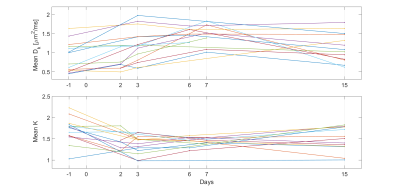
Figure 2: Mean values of the kurtosis-corrected
apparent diffusion coefficient (Dk, upper) and kurtosis (K, lower)
within the tumour as estimated by kurtosis modelling, for all included time
points and mice
Figure 4: Tumour treatment response (TTR) plotted against
estimated mean values of the intracellular volume fraction (fIC), extracellular
extravascular volume fraction (fEES), kurtosis-corrected apparent diffusion
coefficient (Dk), and kurtosis (K) on day 3 relative to day -1, as
well as cell radius (R) on day 3, for all included mice (black dots). Linear
fits are shown as red lines and the Pearson correlation coefficient (r) is shown
in the smaller boxes
Figure 3: VERDICT parameter maps of intracellular
volume fraction (fIC), extracellular extravascular volume fraction (fEES),
vascular volume fraction (fVASC), and cell radius (R), in the central slice of
an example tumour at days -1, 2, 3, 7, and 15. The mouse was treated with external
radiation therapy on day 0