Gayatri Sharma1, Abdul K Abdul K. Parchur2, Jaidip M. Jagtap2, brian M. Fish2, Bergom Carmen 3, Michael Flister4, Meetha M. Medhora5, and Amit Joshi1,6
1Biomedical Engineering, Medical College of Wisconsin, MILWAUKEE, WI, United States, 2Medical College of Wisconsin, MILWAUKEE, WI, United States, 3Radiation Oncology, Medical College of Wisconsin, MILWAUKEE, WI, United States, 4Physiology, Medical College of Wisconsin, MILWAUKEE, WI, United States, 5Medical College of Wisconsin, Medical College of Wisconsin, MILWAUKEE, WI, United States, 6Biophysics, Medical College of Wisconsin, MILWAUKEE, WI, United States
Synopsis
X-ray CT or MR Image-guided radiation therapy (IGRT) is routinely
employed for oral cancer treatment. Enhancing tumor dose while limiting
collateral damage to salivary glands is of critical importance for oral cancer
patients. Here, we demonstrate the
efficacy of multifunctional Theranostic nanoparticles (TNPs) for MR
image guided radiation therapy in a rat xenograft model of oral cancer. These TNPs composed
of Gold (Au) core and Gd(III) shell with combined X-ray and MR contrast can enable
pre-procedure radiotherapy planning via T1-MR imaging, as well as enhance
radiation treatment efficacy by increasing the tumor deposited radiation dose.
Abstract
Introduction: Oral squamous cell carcinoma (OSCC) ranks as one of the common
cancers in the world1. OSCC develops from the epithelium of the oral
cavity, including the tongue, lips, and floor of the mouth, cheeks, hard
palate, or other unspecified parts of the mouth. The surgical resection of the primary
tumor is a standard approach to the treatment of oral cancers, which is most
commonly followed by adjuvant chemotherapy and/or radiotherapy depending on the
risk features or for locally advanced disease. Adjuvant chemo and/or
radiotherapy provide a significant survival advantage in the presence of
positive margins and/or lymph node spread2. However, appropriate
target selection is still a concern in the adjuvant management of oral cavity
carcinoma. There is a need to decide a target and the dose which can minimize the
risk of marginal recurrences and at the same time do not cause toxic effects to
sensitive healthy tissues which affects the quality of life of patients. Nanotechnology
offers to enhance the dose of radiation at the tumor region by using
radiosensitizing nanoparticles. These radiation enhancing agents increase the
effect of radiation in the tumor region while maintaining clinical constrains
on the healthy tissue. Here, we report
the use of 100nm theranostic nanoparticles (TNPs) with combined MR contrast
that can enable pre-procedure radiotherapy planning, as well as enhance
radiation treatment efficacy.
Methods: TNPs were
synthesized by the method as previously published by us3. TNPs
composed of NIR plasmon-resonant core (GNRs) and a Gd (III) inorganic layer as
the shell. Au core was first synthesized using a seed-mediated growth process4
followed by sodium oleate coating at 80°C for 1 h. Uniform Gd (III) shell was
achieved in the presence of hexamethylenetetramine at 120 °C for 3 h. These
TNPs were surface functionalized with –NH2 and then conjugated with mPEG5k-COOH
to obtain a neutral surface charge. These TNPs have both X-ray and MR contrast. TNS were calibrated for X-ray contrast at
60 kV on a Pxinc’s X-RAD SmART scanner using a cone beam CT. MR contrast was
determined on a Brukur 9.4T small animal and GE 7T human scanners. Human oral
squamous cancer cell (OSC-19-GFP-luc) were orthotopically implanted in the
tongue of immune compromised rats. The efficacy of TNPs in enhancing radiation
therapy response was tested via systemic (tail vein) delivery (1μL/g of 1*1013
TNPs). Rats bearing tumors were randomized to saline+radiation (n=4) or TNPs+radiation
(n=5) groups. 8-Gy single dose radiation under MRI guidance was provided 4h
post tail vein injection. Rats were followed via bioluminescence imaging for 4
weeks.
Results: PEGylated
TNPs demonstrated both X-ray and MR contrast in a dose linearly dependent manner.
The T1 relaxivity at 9.4T was found to be ~1.1x108 mM–1S–1
in terms of TNPs concentration. The average TNP size was 75 nm and zeta
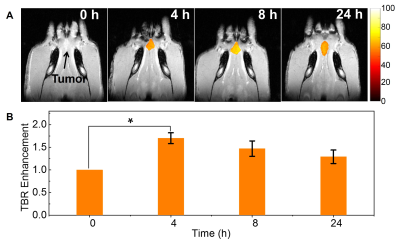
potential ~4.8 mV indicating long circulation potential. Tumors were clearly delineated in MRI images after
i.v. injection of TNPs (Fig. 1A). MRI images showed optimal tumor-to-background
ratio at 4h post injection (Fig. 1B). Following image guided radiation therapy
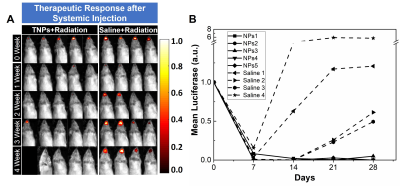
(IGRT) treatment, tumors in TNPs treated rats' experienced reduced tumor growth
(Fig. 1C and D) while rats treated with radiation alone increased tumor growth.
After 4 weeks of follow up, lung metastasis was observed in rats treated with
radiation alone while lungs were clear in TNPs treated rats’. These results
indicate the therapeutic efficacy of TNPs in combination with IGRT.
Discussion: TNPs allow an accurate demarcation of the tumor and also
increase efficiency of radiation treatment.
Conclusions: TNPs with combined MR contrast can enable
pre-procedure radiotherapy planning, as well as enhance radiation treatment
efficacy.
Summary
We report the use of 100nm theranostic nanoparticles (TNPs)
with combined MR and X-ray contrast that can enable pre-procedure radiotherapy
planning, as well as enhance radiation treatment efficacy.Acknowledgements
The authors thank the Alliance for Healthy Wisconsin
and Rock River Cancer Research Foundation (RRCRF, A.J.) for support. References
1. Bray
F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics
2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 2018; 68(6):394-424.
2. Metcalfe E, Aspin L, Speight R, Ermiş E, Ramasamy S, Cardale K, Dyker KE, Sen M, Prestwich RJ.
Postoperative (Chemo) Radiotherapy for Oral Cavity Squamous Cell
Carcinomas: Outcomes and Patterns of Failure. Clin Oncol (R Coll
Radiol). 2017;29(1):51-59.
3. Parchur
AK, Sharma G, Jagtap JM, Gogineni VR, LaViolette PS, Flister MJ, et al.
Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal
Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano. 2018; 12 (7),
pp 6597–6611.
4. Nikoobakht
B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using
seed-mediated growth method. Chemistry of Materials. 2003; 15: 1957-62.