4839
MRI Guided and Enhanced Radiosensitization by MnO2/Pt Nanosheets with Tumor Microenvironment-Triggered in Cancer Radiotherapy1Henan Provincial People’s Hospital, Zhengzhou, China
Synopsis
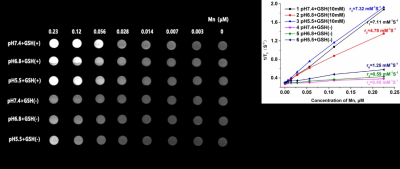
We design the MnO2/Pt system to enhance the radiosensitization. 1-2 nm of platinum are uniformly dispersed on the as-prepared MnO2 nanosheet. The as-prepared MnO2/Pt are degraded by H2O2 and GSH into magnetic resonance functional imaging materials Mn2+. Moreover, MnO2/Pt can degrade GSH to GSSG, which is obstructed DNA repair of cancer cells after radiotherapy. From the results of colony formation, MnO2/Pt can improve the radiotherapy effect on U87 cells with the treatments of X-ray (8 Gy). MnO2/Pt are expected to achieve integrated magnetic resonance guided sensitization radiotherapy in vivo.
Acknowledgements
This research was supported by the National Key R&D Program of China (2017YFE0103600), National Natural Science Foundation of China (81720108021, 81601466), and Zhongyuan Thousand Talents Plan Project-- Basic Research Leader Talent (ZYQR201810117)References
[1] Makale, M. T., McDonald, C. R., Hattangadi-Gluth, J. A., & Kesari, S. (2017). Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nature Reviews Neurology, 13(1), 52.
[2] Donche, S., Verhoeven, J., Descamps, B., Bolcaen, J., Deblaere, K., Boterberg, T., ... & Goethals, I. (2019). The path toward PET-guided radiation therapy for glioblastoma in laboratory animals: a mini review. Frontiers in medicine, 6.
[3] Schiff, D., Desjardins, A., Cloughesy, T., Mikkelsen, T., Glantz, M., Chamberlain, M. C., ... & Wen, P. Y. (2016). Phase 1 dose escalation trial of the safety and pharmacokinetics of cabozantinib concurrent with temozolomide and radiotherapy or temozolomide after radiotherapy in newly diagnosed patients with high‐grade gliomas. Cancer, 122(4), 582-587.
[4] Zhang, Y., Huang, F., Ren, C., Liu, J., Yang, L., Chen, S., ... & Liu, Q. (2019). Enhanced Radiosensitization by Gold Nanoparticles with Acid-Triggered Aggregation in Cancer Radiotherapy. Advanced Science, 6(8), 1801806.
[5] Liu, F., Lin, L., Zhang, Y., Wang, Y., Sheng, S., Xu, C., ... & Chen, X. (2019). A Tumor-Microenvironment-Activated Nanozyme-Mediated Theranostic Nanoreactor for Imaging-Guided Combined Tumor Therapy. Advanced Materials, 31(40), 1902885.
[6] Lin, L. S., Song, J., Song, L., Ke, K., Liu, Y., Zhou, Z., ... & Niu, G. (2018). Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2-Based Nanoagent to Enhance Chemodynamic Therapy. Angewandte Chemie International Edition, 57(18), 4902-4906.
Figures
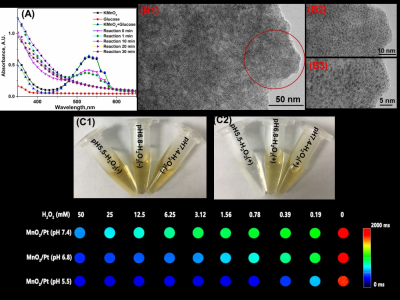
Fig 1. The reaction process monitoring of MnO2/Pt (A) and the TEM image of the MnO2/Pt system at the different magnification(B1-B3), the photos of MnO2/Pt after treatment with different pH buffer solution and/or not H2O2 (C1-C2),and T1 Mapping MRI imaging of MnO2/Pt with different concentration of H2O2 treated (C3) .