4834
MR-guided-radiotherapy gating using 3D positional probability volume derived from time-resolved volumetric MRI: A conceptual study1Medical Physics and Research Department, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
Synopsis
In this study, we proposed a conceptual MRgRT gating strategy using the organ’s motion positional probability volume (PPV) derived from the time-resolved volumetric MRI, aiming to improve treatment accuracy and efficiency. We demonstrated it for the scenario of kidney MRgRT using prospectively acquired CAIPIRINHA-accelerated VIBE MRI data at 1.5T from 7 healthy volunteers. Gating efficiencies of the proposed method were compared to those using the conventional 40%-60% respiratory phase gating. The results showed that the proposed method achieved significantly higher gating efficiency with similar target positional accuracy, indicating its potential value in the future individualized adaptive MRgRT.
Introduction
Respiratory phase/amplitude gating has been widely used for radiotherapy (RT to synchronize the treatment with respiratory motion [1]. With the recent introduction of MR-guided-radiotherapy (MRgRT), such as MRI-LINAC (MRL), it becomes possible to conduct treatment gating based directly on real-time acquired MR images instead of the traditional external respiratory surrogates or implanted fiducial markers [2, 3], while the gating algorithm is mostly inherited from the traditional ones. However, current online MRgRT gating is mostly based on a single slice or a limited number of slices (single or orthogonal views) [4]. It might substantially under- or over-estimate organ motion and thus affect gating accuracy and efficiency. In this study, we proposed a conceptual MRgRT gating strategy using the organ’s motion positional probability volume (PPV) derived from the time-resolved volumetric MRI, aiming to improve treatment accuracy and efficiency.Methods
We demonstrated this proposed gating method for the scenario of kidney MRgRT using prospectively acquired data from 7 healthy volunteers. They received free-breathing MRI with RT positioning on a 1.5T MRI-simulator (Siemens Healthineers, Erlangen, Germany). A 3D CAIPIRINHA-accelerated VIBE sequence [5] was used for acquisition (transversal, FOV=350mmx262.5mm, thickness=4mm, matrix size=128x128x56, TE/TR=0.6/1.7ms, flip-angle=6o, RBW=1250Hz/voxel, CAIPIRINHA factor=4, partial Fourier-factor=6/8, temporal resolution = 1.0frame-per-second). Two MRI scans lasted 144s and 576s without volunteer re-positioning.The dataset of the first scan was used to generate the on-the-day PPV. Two kidneys were delineated on the first timeframe to create reference position and reference volume (Vk). Images of following timeframes were linearly registered to the references to create the dynamic renal binary masks. Voxel-wise renal positional probability map (PPM) was calculated by the number of frames that a voxel had been occupied by kidney divided by the elapsed timeframes, i.e. PPMj=(M1+...+Mj)x100%/j. (binary mask value Mj=1 or 0, j: timeframe index). Positional probabilistic volume with PPM≧i% (Vi%) could be calculated dynamically at every timeframe. For gating purpose, renal Vi% (i=0, 2.5, 5, 7.5,10) was calculated using all 144 timeframes.
The second scan dataset was used to simulate MRgRT treatment procedure and assess gating performance. The moving kidney volume (Vd) of each timeframe was determined either by segmentation on the present images or registering to the reference position. If the concordance index (CIj) of timeframe j, calculated by (Vd∩Vi%)/(Vd∪Vi%), exactly equalled to Vd/Vi% (i.e. Vd was totally within the Vi%), gating was triggered on to deliver radiation (beam-on). Otherwise, irradiation was stopped (beam-off). Gating efficiency (Eg), or duty cycle, calculated by the duration of beam-on time divided by the total scan/treatment duration (576s), was calculated for different Vi%. These gating efficiencies were compared to those using the conventionally adopted gating window of 40%-60% respiratory phase, determined by liver dome.
Results
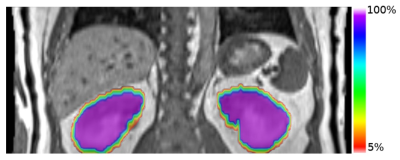
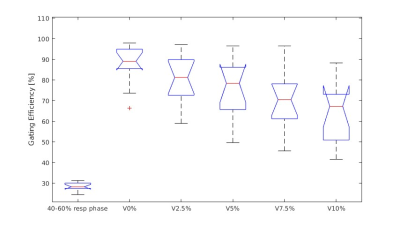
The cohort’s renal reference volume was 190±42 mm3 and 174±34mm3 for left and right kidney. The calculated Vi% (i=0, 2.5, 5, 7.5, 10) was 246±48mm3, 233±50mm3, 230±50mm3, 226±47mm3 and 221±43mm3, for left kidney, and 218±41mm3, 211±41mm3, 208±39mm3, 206±38mm3 and 204±38mm3 for right kidney. V0% of a volunteer overlaid on the reference position image was illustrated (Fig. 1). Gating efficiency Eg was 87±8%, 77±13%, 72±15%, 68±16%, and 66±14% for left kidney, and 88±12%, 83±12%, 78±15%, 72±13% and 62±16% for right kidney, respectively, when V0%, V2.5%, V5%, V7.5% and V10% was used for gating. No significant difference in Eg between two kideneys for all Vi%. Compared with Eg by using 40%-60% respiratory phase (28±2%), PPV Eg was significantly higher (p<0.01, Wilcoxon signed-rank test) in both kidneys for all Vi% (Fig. 2). An illustration of beam-on and beam-off duration along with the SI displacement of liver dome and kidney in a subject was shown in Fig. 3.Discussion
We proposed a novel gating strategy for online MRgRT by using the time-resolved volumetric MRI derived on-the-day PPV. The results showed that the proposed method achieved significantly higher gating efficiency than the conventional respiratory phase gating, with similar target positional accuracy. Thus, it might hold potentials for the individualized precise MRgRT. Furthermore, on-the-day PPV might be also useful for treatment adaptation if considerable deviation from planning is found. This study has the major limitation of a small sample size of healthy volunteers, in which cohort the respiratory irregularity might be much underestimated compared to the real patient. Thus the gating efficiency might be overestimated. The linear image registration for PPV generation did not account for the organ deformation during respiration, and might affect Vi% accuracy. Dose evaluation was not conducted for demonstration of dose advantage due to the nature of volunteer study design. Much work is warranted for real clinical use. First, image reconstruction latency to acquisition must be minimized, aiming for real-time imaging. Meanwhile, target segmentation (and/or registration) on the newly acquired images and volumetric computation should be fast enough for gating judgement. Synchronization and interaction between imaging and accelerator is for sure to be developed.Acknowledgements
This study was approved by the Institutional Research Ethics Committee (REC-2019-09)References
[1]. Yoganathan SA, Maria Das KJ, Agarwal A, Kumar S. Magnitude, Impact, and Management of Respiration-induced Target Motion in Radiotherapy Treatment: A Comprehensive Review. Journal of medical physics. 2017;42(3):101-15.
[2]. Lagendijk JJ, Raaymakers BW, Van den Berg CA, Moerland MA, Philippens ME, van Vulpen M. MR guidance in radiotherapy. Phys Med Biol. 2014;59(21):R349-69
[3]. Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24(3):207-9.
[4]. Seregni M, Paganelli C, Lee D, Greer PB, Baroni G, Keall PJ, et al. Motion prediction in MRI-guided radiotherapy based on interleaved orthogonal cine-MRI. Phys Med Biol. 2016;61(2):872-87.
[5]. Yuan J, Wong OL, Zhou Y, Chueng KY, Yu SK. A fast volumetric 4D-MRI with sub-second frame rate for abdominal motion monitoring and characterization in MRI-guided radiotherapy. Quant Imaging Med Surg. 2019;9(7):1303-14.
Figures