4822
DWI-based Radiomics Nomogram Predicting Recurrence-Free Survival in Patients with Muscle-Invasive Bladder Cancer
Shan Zhang1, Guangyu Wu1, Guiqin Liu1, Yongming Dai2, and Yunfei Zhang2
1Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China, 2United Imaing Healthcare, Shanghai, China
1Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China, 2United Imaing Healthcare, Shanghai, China
Synopsis
We hypothesized that the radiomics features obtained from DWI holds great potential in improving the recurrence risk stratification of MIBC patients. Thus, we developed a radiomics nomogram and compared its performance with clinicopathological nomogram and radiomics signature in individual RFS prediction. Our results showed that the radiomics nomogram, which incorporates radiomics signatures, clinical characteristics and molecular characteristics, has greater potential to serve as a biomarker to estimate the RFS in MIBC patients. In conclusion, a radiomics nomogram may serve as a potential tool to facilitate individualized prediction of recurrence in patients with MIBC.
INTRODUCTION
Prediction of the recurrence or metastasis risk is significant for prognostication and individualized management of patients with muscle-invasive bladder cancer (MIBC). Diffusion-weighted imaging (DWI) is a potential MRI technique to predict the clinical outcome. 1-3 Moreover, it has been widely reported that radiomics is an powerful technique to convert medical images into mineable high-dimensional quantitative data through feature extraction and machine learning techniques4,5. Thus, this research aims to develop a radiomics nomogram utilizing DWI in order to predict recurrence-free survival (RFS) in MIBC patients and to assess its incremental value over traditional staging system and clinicopathological risk factors for individual RFS prediction.METHODS
In this retrospective study, 210 MIBC patients undergoing preoperative DWI were enrolled and randomly divided into training (n=105) and validation (n=105) cohorts. An eight-feature radiomics signature was built with LASSO model from training cohort6. Association between the radiomics signatures and RFS was evaluated. A radiomics nomogram was generated to assess the incremental value of the radiomics signature in individual RFS estimation in terms of calibration, discrimination, reclassification and clinical usefulness.RESULTS
The radiomics signatures were significantly associated with RFS in both training and testing cohorts (log-rank p<0.01) and was independent with clinicopathological factors (p<0.05) (Figure 1). The radiomics nomogram achieved better performance in DFS prediction (C-index: 0.702, 95% confidence interval [CI]: 0.602, 0.802) than both clinicopathological nomograms (C-index: 0.682, 95% CI: 0.575, 0.788) and radiomics signature only (C-index: 0.612, 95% CI: 0.493, 0.731), and achieved better calibration and classification of survival (net reclassification improvement: 0.226, 95% CI: 0.016, 0.415, p=0.038) (Figure 2). Decision curve analysis suggested the better predictive performance can be obtained with such strategy (Figure 3).DISCUSSION
This study demonstrated that the radiomics signature can be used to estimate the RFS. Moreover, incorporating the radiomics signatures, clinical characteristic and molecular characteristics, a radiomics nomogram was able to achieve much greater prognostic performance than either the radiomics signature or the clinical-pathological nomogram with a better discrimination, calibration and clinical usefulness. This indicated that the radiomics nomogram added the incremental value to clinical-pathological nomogram for individualized estimation.CONCLUSION
The DWI-based radiomics signature was an independent predictor of RFS in patients with MIBC. Combining the radiomics signatures, clinical staging and other established risk factors achieved better performance in individual RFS prediction. For precision medicine, such a radiomics nomogram model of MIBC may potentially be useful, although this will require further external validation before extensive clinical implementation.Acknowledgements
References
1.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007;188:1622-35. 2. Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11:102-125. 3. Yoshida S, Koga F, Kobayashi S, et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys 2012;83:e21-7. 4. Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:4006. 5. Gillies RJ, Kinahan PE, Hricak H et al. Radiomics: Images are more than pictures, they are data. Radiology 2016;278:563-577. 6. Tibshirani R. The lasso method for variable selection in the Cox model. Statistics in Medicine, 1997, 16(4):385-395.Figures

Figure 1.
(a) Kaplan-Meier curves of RFS in the training
data set stratified by the high- and low-risk group. (b) Kaplan-Meier curves of
RFS in the testing data set stratified by the high- and low-risk group.
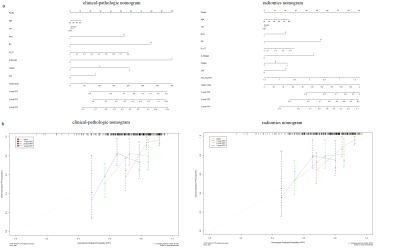
Figure 2.
Clinical-pathologic nomogram and radiomics nomogram to estimate the RFS
(a) and the assessment of model calibration (b).
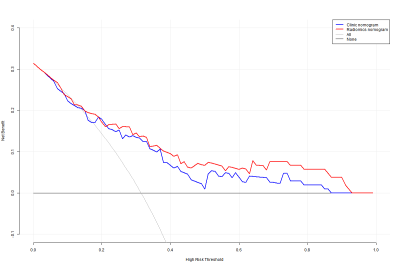
Figure 3. Decision curve analysis (DCA) for
radiogenomics nomogram and clinical-pathologic nomogram to estimate the and RFS.
The x-axis represents the threshold probability and the y-axis measures the net
benefit