4819
A deep learning model to predict near pathological complete response for rectal cancer by the diffusion MRI data before chemoradiotherapy
Hai-Tao Zhu1, Xiao-Yan Zhang1, Yan-Jie Shi1, Xiao-Ting Li1, and Ying-Shi Sun1
1Peking University Cancer Hospital, BEIJING, China
1Peking University Cancer Hospital, BEIJING, China
Synopsis
A deep learning model is proposed to predict near pathological complete response by diffusion MRI data before chemoradiotherapy. 624 participants are included in this study with 424 for training and 200 for testing. The area under the curve of receiver operating characteristic is 0.800 (95%CI: 0.735-0.851). The sensitivity is 0.700 (95%CI: 0.568-0.812), the specificity is 0.871 (95%CI: 0.804-0.922). Compared with the strategy that uses both pre-NCRT and post-NCRT data, the method may predict the pathological results at an earlier time point before the initiation of NCRT, which enables a chance to modify the NCRT plan if needed.
Introduction
Preoperative neoadjuvant chemoradiotherapy (NCRT) combining total mesorectal excitation (TME) is the standard treatment procedure of locally advanced rectal cancer [1]. Nevertheless, the loss of sphincter function greatly affects the life quality of patients. Recently, “wait and see” policy or local excision has been proposed instead of TME for the patients showing pathological complete response (pCR) (ypT0N0) or near-pCR (ypT0-1N0) [2]. Meanwhile, diffusion MRI has been considered as an effective imaging modality for the prediction of near-pCR by combining machine learning methods such radiomics [3,4]. In these studies, both pre-NCRT and post-NCRT data have been used to construct a quantitative model with predefined features. It is more desirable to predict near-pCR before the initiation of NCRT as it may provide a chance to modify NCRT plan. Furthermore, deep learning makes it possible to extract useful information from the images without the need of feature pre-definition. In this study, a quantitative model is proposed to predict near-pCR by pre-NCRT diffusion MRI data and convolutional neural networks.Methods
This retrospective study enrolled 624 participants with rectal cancer from October 2015 to December 2017. All participants were proven as locally advanced rectal adenocarcinoma by histopathology and baseline MRI examination (≥cT3 or N+). All MRI were performed with a 3.0 Tesla MRI scanner (Discovery MR750) using an 8-channel phased array body coil in the supine position. Diffusion-weighted MRI (DWI) images were obtained by using SSEPI at b-value of 1000 s/mm2 and b=0, FOV is 34cm, TR is 2.8s, TE is minimum, thickness is 4mm, gap is 1mm. Th. Region of interests (ROI) of rectal tumor were manually drawn on each slice that contained tumor. All participants received 22-fraction intensity-modulated radiation therapy. After TME, surgically resected specimens were examined and analyzed by two pathologists in consensus. Pathological results were used as ground truth with near-pCR (ypT0-1N0) labeled as 1 and others labeled as 0. All participants were divided into training set (n=424) and test set (n=200) chronically. DWI images at b=0 and b=1000 s/mm2 are inputted into convolutional neural networks from two separated channels. Each channel passed through 5 repetitions of convolution and max-pooling layers (CMC unit) as is shown in Fig. 1, and the last layer is densely connected. The network architecture was implemented using Python 3.6 based on Keras 2.1.5 with TensorFlow 1.4.0 as its backend. The optimal setting of the hyperparameters were decided by 3-fold cross-validation in the training set. After the determination of hyperparameters, the corresponding network was validated on the test set. Data augmentation was performed by rotating the ROI by N times with each of 360/N degrees. N is 100 for Near-pCR and N is 30 for others according to the ratio of two classes. Stochastic gradient descent algorithm with the adaptive moment estimation algorithm (ADAM) optimizer was used in training with a learning rate of 10-5 and a decay rate of 10-5, a mini-batch size of 30, and a binary cross-entropy loss function. The network was trained for 2000 epochs.Results
Among the 424 participants in the training set, 96 (22.6%) participants achieved near-pCR after chemoradiotherapy. Among the 200 participants in the test set, 60 (30.0%) participants achieved near-pCR after chemoradiotherapy. The predicted probability of near-pCR by the deep learning model was compared with pathological ground truth to draw a receiver operating characteristic (ROC) curve in Fig.2. The area under the curve (AUC) is 0.800 (95%CI: 0.735-0.851). The maximum Youden’s index is used to determine the cutoff value of near-pCR. The sensitivity is 0.700 (95%CI: 0.568-0.812), the specificity is 0.871 (95%CI: 0.804-0.922).Discussions
In this study, we proposed a deep learning method to predict near-pCR by only using the pre-NCRT data. Compared with the strategy that uses both pre-NCRT and post-NCRT data, the method may predict the pathological results at an earlier time point before the initiation of NCRT, which enables a chance to modify the NCRT plan if needed. The study uses two channels of images at b=0 and b=1000 s/mm2 as the input instead of using ADC image for three reasons. First, since all the images were acquired at the same scanner, it is possible to directly compare DWI images. Second, the calculation of ADC image may lead to errors due to distortion or noise. Third, the T2-weighted components in DWI images may contribute additional information to the deep learning model. The main limitation of this single center study is the lack of a true external validation cohort using different MRI scanners and field strength.Conclusion
Near pathological complete response (ypT0-1N0) to chemoradiotherapy for rectal cancer can be predicted by the diffusion weighted images before chemoradiotherapy using deep learning method.Acknowledgements
No acknowledgement found.References
- Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405- Prodige 2. J Clin Oncol 2010; 28: 1638–44
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391 (10139):2537-2545.
- Bulens P, Couwenberg A, Haustermans K, et al. Development and validation of an MRI-based model to predict response to chemoradiotherapy for rectal cancer. Radiotherapy and Oncology 2018, 126(3), 437-442.
- Tang Z, Zhang XY, Liu Z, et al. Quantitative analysis of diffusion weighted imaging to predict pathological good response to neoadjuvant chemoradiation for locally advanced rectal cancer. Radiotherapy and Oncology 2019, 132, 100-108.
Figures
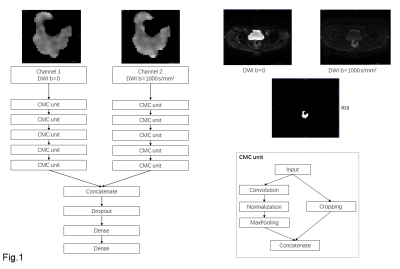
Fig.1: Structure of convolutional neural networks.DWI
images at b=0 and b=1000 s/mm2 are inputted into convolutional neural
networks from two separated channels. Each channel passes through 5 repetitions of convolution and max-pooling layers (CMC unit). The
last layer is densely connected.
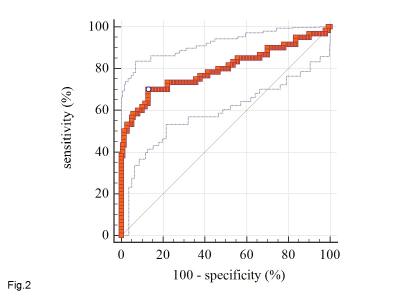
Fig. 2: Receiver
operating characteristic (ROC) curve of the deep learning model. The
area under the curve (AUC) is 0.800 (95%CI: 0.735-0.851).