4813
Antitumor effects of NK-derived nanovesicles in a B-cell lymphoma preclinical model by integrating in vivo MRI and ex vivo MRS1NMR and MRI unit, Core facilities, Istituto Superiore di Sanita', Rome, Italy, 2National Center for animal experimentation and welfare, Istituto Superiore di Sanita', Rome, Italy, 3Microscopy unit, Core facilities, Istituto Superiore di Sanita', Rome, Italy, 4Oncology and Molecular Medicine Department, Istituto Superiore di Sanita', Rome, Italy
Synopsis
NK cells (NK) are the first barrier of body defense from tumor cells. Individuals with low NK activity display an increased risk to develop cancer. Extracellular vesicles are secreted vesicles possessing immune regulatory properties. We demonstrated that exosomes produced by NK cells (isolated from blood of healthy donors) display a cytotoxic activity against tumors in vitro. In this work we monitor the effects of NKEVs treatment by in vivo MRI/MRS and ex vivo MRS metabolomics in a xenograft model of lymphoma. We found altered lipid and redox metabolism suggesting taurine and lipid signals as potential biomarkers of NKEVs response
Introduction
NK cells (NK) are the first effective barrier of body defense from tumor cells. Individuals with low NK activity display an increased risk to develop cancer (1). Exosomes (EXO) and microvesicles (MV) are nanometer-sized secreted vesicles, 30-150 nm and 150-400 nm respectively, involved in numerous biological networks (2,3). The in vivo immune regulatory properties and cell communication of these nanovesicles have been demonstrated in preclinical studies, supporting the possible use of exosomes in diagnosis and therapy of different disease, such as cancer (4). We have demonstrated that exosomes produced by NK cells (EXONK), isolated from blood of healthy donors, display a potent cytotoxic activity against tumors in vitro (5). To date, it has been demonstrated that extracellular vesicles of NK (NKEVs) cells of tumor origin or genetically modified exert an antitumor effect towards murine melanoma (6), neuroblastoma (7) and glioblastoma (8).Aim
The aim of the present work was to monitor the effects of NKEVs treatment by integrating in vivo MRI/MRS and ex vivo MRS metabolomics in a xenograft model of human B-cell lymphomaMethods
Xenografts were derived from s.c. implantation of SUDHL4 B-cell lymphoma in SCID mice, and treated twice per week with NKEVs=EXONK+MVNK (NK-derived extracellular vesicles, both exosomes and microvesicles ) or saline intra tumors (SAL). One group of animals (Co-NKEV) started to receive the treatment during simultaneously with the injection of cancer cells, another group (Post-NKEV) started to receive the treatment a week later. Tumour growth was monitored twice per week by a caliper.In vivo MRI/MRS measurements were performed on a Varian/Agilent Inova system, operating at 4.7 T. MRI/MRS and a combination of volume and surface coil (RAPID Biomedical). MRI evaluation was performed by T1W (TR/TE=500/20ms) and T2W (TR/TE=3000/70ms) multislice spin echo images.
Quantitative in vivo MRS analyses were performed according to a protocol described by (9) by using a PRESS sequence (TR/TE =4000/23 ms), which includes T2 water measurements and LCModel analysis for spectral fitting.
Quantitative MRI ADC measurements were performed by acquiring DW images (TR/TE=2000/50 ms, b ranging from 0 to 1105 s/mm2). D, f and D* parameters were derived from DWI by applying IVIM analysis. Moreover, the different contributions of ADCslow and ADCfast for diffusion and perfusion estimation in tumours have been performed by two separate fits for b values higher or smaller than 100 s/mm2, respectively.
Ex vivo high resolution MRS analyses were performed on tissue extracts at 9.4 by using high resolution Bruker Avance spectrometer as described by (10).
Histological analysis of xenograft sections of specimens collected at the end of the therapy are ongoing.
Results
Tumour growth reduction and MRI results We observed a significant reduction of tumour growth [ANOVA repeated measurement, with treatment (saline, Co-NKEV an Post-NKEV) x time (7 points) design p=0.003] at all-time points starting from day 13 after the injection for the Co-NKEV group and at day 19 for the Post-NKEV group (Tukey post hoc analyses). We do not observed any significant changes in the diffusivity parameters derived from DWI.In vivo MRS results We found in the in vivo MR spectra a significant increase in the tumour lipid/lactate signal at 1.33 ppm (ANOVA one-way, p=0.0015) and a significant increase in taurine (ANOVA one-way, p=0.0018) in the Co-NKEV group with respect to controls and to the Post-NKEV group which could be attributed to cell apoptosis.
Ex vivo MRS results Ex vivo high resolution MRS analyses on organic tumor tissue extracts showed a different composition of lipidome between NKEV –and saline- treated tissues . In particular we found a significant reduction (p <0.05) of degree of unsaturation and an increase of ω-CH3(FA) in Co-NKEV (n=6) as compared to the saline-treated control tissues (n=5). We also observed the same differences in the degree of unsaturation (UFA and MUFA) between Post-NKEV(n=7) and control groups; p=0.05. No differences were found in the levels of triacylglycerols (TAG), phosphatidylcholine (PC)+LysoPC and total cholesterol between both treated and control tumors.
Discussion and Conclusion
In vivo MRI/MRS and ex-vivo HR-MRS showed previously unexplored NKEVs induced metabolic changes in a model of human B-cell lymphoma suggesting taurine and lipid signals as potential biomarkers of NKEVs response which provide evidence of altered lipid and redox metabolism following antitumor NKEV treatment.Acknowledgements
We acknowledge partial support by a grant of Ministry of Health (GR-2011-02351400 to LL).References
1. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9(5):503-510.
2. Bakhshandeh B, Kamaleddin MA, Aalishah K. A Comprehensive Review on Exosomes and Microvesicles as Epigenetic Factors. Curr Stem Cell Res Ther 2017;12(1):31-36.
3. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009;9(8):581-593.
4. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 2018;15(10):617-638.
5. Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, Paris L, Abalsamo L, Colone M, Molinari A, Podo F, Rivoltini L, Ramoni C, Fais S. Immune surveillance properties of human NK cell-derived exosomes. J Immunol 2012;189(6):2833-2842.
6. Lee EY, Park KS, Yoon YJ, Lee J, Moon HG, Jang SC, Choi KH, Kim YK, Gho YS. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLoS ONE 2012;7(3):e33330.
7. Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY, Seeger RC, Fabbri M. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res 2018;79(6):1151-1164.
8. Zhu L, Oh JM, Gangadaran P, Kalimuthu S, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. Targeting and Therapy of Glioblastoma in a Mouse Model Using Exosomes Derived From Natural Killer Cells. Front Immunol 2018;9:824.
9. Canese R, Pisanu ME, Mezzanzanica D, Ricci A, Paris L, Bagnoli M, Valeri B, Spada M, Venditti M, Cesolini A, Rodomonte A, Giannini M, Canevari S, Podo F, Iorio E. Characterisation of in vivo ovarian cancer models by quantitative 1H magnetic resonance spectroscopy and diffusion-weighted imaging. NMR Biomed 2012;25(4):632-642.
10. Pisanu ME, Ricci A, Paris L, Surrentino E, Liliac L, Bagnoli M, Canevari S, Mezzanzanica D, Podo F, Iorio E, Canese R. Monitoring response to cytostatic cisplatin in a HER2(+) ovary cancer model by MRI and in vitro and in vivo MR spectroscopy. Br J Cancer 2014;110(3):625-635.
Figures
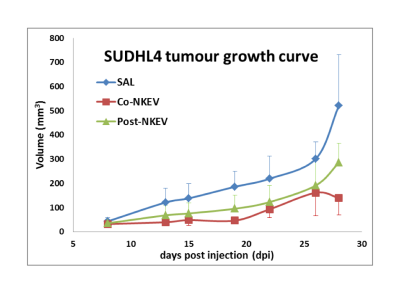
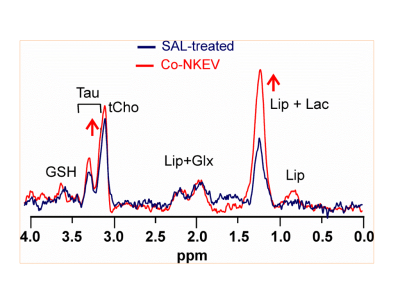
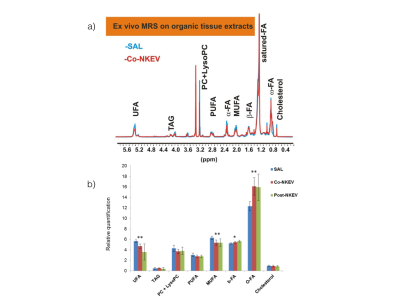
Figure 3 - a) 1H HR-NMR spectra acquired from Co-NKEV and SAL-treated on organic tissue extracts. b) Histograms represent the relative quantification of lipid metabolites between NKEV- and saline-treated tumor tissues. Peak assignment: UFA, unsaturated fatty acid p=0.01; MUFA monounsaturated fatty acid (FA) p=0.01; O-CH3(FA; 0.89 ppm) p=0.01, b-CH3(FA; 1.56 ppm) p=0.01. p value is referred to significant differences between NKEV-treated and Saline control groups