4810
Quantification of Fructose Metabolism in Hepatocellular Carcinoma1Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Fructose metabolism utilizes a distinct set of transporters and enzymes and is limited to the liver, kidney and small intestines. Key to the ability to break down fructose is the enzyme ketohexokinase (KHK), that phosphorylates fructose to fructose-1-phosphate (F1P). In this study, we demonstrate that fructose metabolism is downregulated in a mouse model of hepatocellular carcinoma (HCC). Based on this novel observation, we use 13C nuclear magnetic resonance (NMR) to quantify altered hepatic fructose metabolism. We demonstrate significantly decreased F1P production in HCC, providing justification for developing non-invasive methods to detect fructose metabolism.
Introduction
While glucose and fructose are both six-carbon sugars, these metabolites are broken down in distinct tissues using a complement of different transporters and enzymes. Fructose uptake is facilitated by cell surface transporters such as, GLUT2 is the predominant transporter for fructose in the liver, allowing bi-directional transport with low affinity1. In the cell, fructose is phosphorylated to form fructose-1-phospohate (F1P), catalyzed by KHK2. F1P is further metabolized by aldolase B (Aldo-B) that catalyzes the specific and reversible cleavage of hexoses. While fructose metabolism has been implicated in metabolic disease, the fate of fructose in HCC is unknown. This study investigates the fate of fructose in liver cancer, with a focus on developing quantitative methods to detect fructolysis for early detection and monitoring of HCC.Materials and Methods
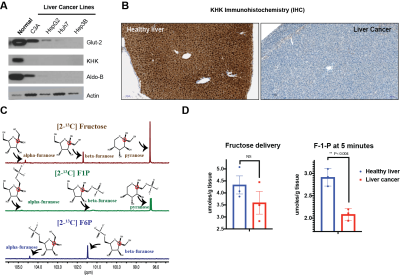
C3A, HepG2, Huh7 and Hep3B cells were grown under standard cell culture conditions. Cells were lysed in RIPA and used for Western blot analysis. Immunohistochemistry (IHC) was performed in formalin-fixed, paraffin-embedded slices of a mouse model of HCC3, using a 1/200 dilution of anti-KHK primary antibody (Sigma Aldrich). The same mouse model was infused with [2-13C] fructose (Sigma Aldrich) via tail vein injection. After euthanasia, the liver was harvested and extracted for 13C NMR.Results
Western blots of normal liver demonstrate robust expression of fructose transporter GLUT2 as well as the enzymes KHK and Aldo-B. However, all other liver cancer cells demonstrated dramatically lower levels of proteins involved in fructose metabolism as shown in Figure 1A. IHC of a mouse models of HCC showed similar trends, where KHK expression is dramatically lower in tumor regions compared to normal liver (Figure 1B). We proceeded to investigate if 13C NMR would be able to distinguish the fates of different metabolites of fructose. As depicted in Figure 1C, different anomers of fructose and associated metabolites displayed distinct chemical shifts. Therefore, we proceeded to demonstrate that fructose flux is also compromised as a consequence of KHK expression in vivo. We infused [2-13C] fructose in a mouse model of HCC. While delivery of fructose to the liver of HCC mice were lower than healthy mice (3.58 ± 0.81 vs. 4.33 ± 0.65 µmoles/g tissue), this difference was not significant. However, F1P levels were significantly lower in HCC mice (2.07 ± 0.12 µmoles/g tissue) compared to healthy mice (2.91 ± 0.19 µmoles/g tissue). These results confirm that NMR-based methods can be used to quantify changes in fructose metabolism in HCC.Discussion and Conclusions
This study has demonstrated metabolic rewiring in HCC, with the entire program of fructose metabolism being downregulated in a mouse model of liver cancer. The majority of fructose that is broken down in the liver is used for gluconeogenesis, to produce glucose for other cells and tissues. As KHK activity is dependent on the availability of adenosine triphosphate (ATP), cancer cells have adapted a different metabolic program that does not favor ATP use for fructose breakdown. This ATP can then be routed for rapid cell growth and division that is a hallmark of cancer. This metabolic rewiring can be detected by 13C MRS – by quantifying the F1P produced in the liver. We posit that non-invasive methods to detect this metabolic program in the liver can be useful for early detection of HCC as well as monitoring disease progression. In the future, we propose using hyperpolarized MRS to quantify the rate of KHK activity, as a translational tool to detect hepatic fructose metabolism in liver cancer.Acknowledgements
The authors acknowledge the following funding sources : The Tow Foundation, Starr Cancer Consortium, Stand Up to Cancer, Geoffrey Beene Cancer Research Center, and the Center for Molecular Imaging and Nanotechnology at MSKCC. The authors also thank Mojdeh Shakiba from Dr Andrea Schietinger’s lab for providing the mouse model and the Sloan Kettering Institute Molecular Cytology core for IHC.References
1 Douard, V. & Ferraris, R. P. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295, E227-237, doi:10.1152/ajpendo.90245.2008 (2008).
2 Cori, G. T., Ochoa, S., Slein, M. W. & Cori, C. F. The metabolism of fructose in liver; isolation of fructose-I-phosphate and inorganic pyrophosphate. Biochim Biophys Acta 7, 304-317 (1951).
3 Schietinger, A. et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 45, 389-401, doi:10.1016/j.immuni.2016.07.011 (2016).
Figures