4806
Polyunsaturated fatty acids (PUFA) depletion in high serotonin turnover breast tumours using advanced magnetic resonance spectroscopy (MRS)1Aberdeen Biomedical Imaging Centre, University of Aberdeen, Aberdeen, United Kingdom, 2Pathology Department, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 3Breast Unit, Aberdeen Royal Infirmary, Aberdeen, United Kingdom, 4Institute of Pharmacy and Biological Sciences, University of Strathclyde, Glasgow, United Kingdom
Synopsis
Locally advanced breast cancer is the most common cause of death in middle-aged women, with research focus shifted to preventative medicine. Polyunsaturated fatty acids (PUFA) is depleted in tumour initiation to sustain an inflammatory tumour microenvironment conducive to macrophage recruitment. Serotonin modulates macrophage activity and is a marker of poor 10-year survival in women with breast cancer. The relationship between serotonin and PUFA demands close examination for preventative treatment optimisation. We applied high sensitivity double quantum filtered MRS to accurately quantify PUFA between serotonin low and high breast tumours, and found PUFA depletion is associated with increased serotonin turnover.
Introduction
Breast cancer is the most common cause of cancer-related death among women1, with research focus increasingly shifted to disease prevention and personalised medicine2. During tumour initiation, the conversion from polyunsaturated fatty acids (PUFA) to inflammatory agent of eicosanoids is increased3, stimulating tumour necrosis factor-α (TNFα) and macrophages to sustain an inflammatory tumour microenvironment4. Elevated inflammation is associated with increased serotonin turnover linked to poor 10-year survival rate5. Earlier evidence showed that PUFA deficiency increased serotonin turnover, however it was evaluated in animal brain models6. Specialist double quantum filtered (DQF) magnetic resonance spectroscopy (MRS) allows accurate detection of PUFA, while overlapping water and lipid signals are suppressed7. We hypothesised that there is an association between PUFA from whole breast tumours and serotonin expression.Methods
Thirty female patients (age 39 – 78 years, 12 serotonin ≤100 and 18 serotonin >100, all invasive ductal in type) were included in the study. Only patients over the age of 18, with a tumour size larger than 1 cm in diameter on ultrasound and undergoing wide local excision or mastectomy were eligible. Patients with previous breast malignancies, received neoadjuvant chemotherapy or neoadjuvant hormonal therapy, or with conditions contraindicated in MRI were not eligible. The study was approved by the North West – Greater Manchester East Research Ethics Committee (REC Ref: 16/NW/0032), and written informed consent was obtained prior to the MRS study (Figure 1).MRS Acquisition
Immediately after excision of the tumour specimen, the fresh tissue (without formalin treatment) was immediately transported to the imaging centre for MRS scan. All data were acquired on a 3.0 T whole body clinical MRI scanner (Achieva TX, Philips Healthcare, Best, Netherlands) using a body coil for uniform transmission and a 32-channel receiver coil for high sensitivity detection. T1-weighted anatomical images were acquired using standard 3D sequence8 with matrix size of 220 × 220, an isotropic voxel size of 1 mm, echo time (TE) of 2.7 ms, repetition time (TR) of 5.2 ms, and imaging volume encompassing the whole specimen. The PUFA spectrum was acquired from a single voxel snug fit to the tumour using DQF MRS method7 with TR/TE of 1.25 s/130 ms, spectral editing frequency at 2.8 ppm, bandwidth of 2000 Hz and 1024 points. A reference spectrum was acquired from the same voxel using PRESS sequence9 with TR/TE of 1.25 s/130 ms.
Data Processing
The reference and PUFA spectra were pre-processed following standard procedures10 and quantified using AMARES algorithm11 in the jMRUI software (v3.0, TRANSACT, Leuven, Belgium)12. Methyl fat (0.9 ppm) and PUFA (5.3 ppm) amplitudes were quantified from the reference and DQF MRS spectra respectively using corresponding prior knowledge databases13, with PUFA expressed as the percentage ratio between PUFA and methyl fat.
Histopathological Analysis
Standard histopathological examination was undertaken to determine tumour size, grade, lymph node metastasis and Nottingham Prognostic Index (NPI)14. Immunostaining was conducted in a single batch for tumour proliferative marker Ki-6715 and serotonin. The semiquantitative H-score16 was used for serotonin, with the final score ≤100 classified as low expression, and the score >100 as high expression.
Statistical Analysis
Statistical analysis was performed in the SPSS software (Release 23.0, SPSS Inc., Chicago, IL, USA). T-test was performed on normally distributed PUFA to assess the group difference between serotonin low and high tumours, and Mann Whitney U test for non-normally distributed Ki-67 expression. Correlation tests were performed between PUFA against serotonin and Ki-67 expression in the entire cohort. A p value <0.05 was considered statistically significant.
Results
The patient demographics are summarised in Table 1. There were no significant differences in age, body mass index and tumour size between low and high serotonin groups. There was a significant higher PUFA (t = 2.197, p = 0.0442) in low serotonin (1.45 ± 0.81 %) breast tumours than high serotonin (0.90 ± 0.42 %) (Table 2, Figure 2a). There was no significant difference in Ki-67 expression (z = 1.820, p = 0.0687) between low serotonin (median of 21.7 %, interquartile range (IQR): 12.3 – 39.7 %) and high serotonin (median of 14.5 %, IQR: 7.8 – 18.7 %) breast tumours (Table 2, Figure 2b). PUFA was negatively correlated with serotonin expression (r = -0.3616, p = 0.0496, Table 2, Figure 3a), but not with Ki-67 expression (p = 0.2721, Table 2, Figure 3b).Discussion
We found that PUFA was higher in whole breast tumours with low serotonin expression compared to high serotonin. There was a negative correlation between PUFA and serotonin expression. Our results were in agreement with previous pre-clinical studies showing the direct link between serotonin and PUFA6 through the mediating pathways of eicosanoids3,17, TNFα18,19 and IL-620. The current study is the first attempt to assess PUFA in low and high serotonin whole breast tumours and supports PUFA and inflammation evaluation in human breast cancer.Conclusion
Our results showed that there is a significant reduction in PUFA in high serotonin breast tumours. PUFA in human breast cancer is associated with serotonin mediated through the inflammation process, offering a potential target for preventative treatment optimisation.Acknowledgements
The authors would like to thank Dr Matthew Clemence for clinical scientist support, Ms Dawn Younie for logistic support, Prof Andrew M Blamire for advice on MRS, Ms Mairi Fuller and Mr Roger Bourne for providing access to patients. This project was funded by Friends of Aberdeen and North Centre for Haematology, Oncology and Radiotherapy (ANCHOR), and Sai Man Cheung was jointly supported by Elphinstone scholarship, Roland Sutton Academic Trust and John Mallard scholarship.References
1. Office of National Statistics (ONS). Causes of Death Over 100 Years. London: Office of National Statistics; 2017:1-8.
2. Breast Cancer Now. About our research. Available from: http://breastcancernow.org/breast-cancer-research/about-our-research. Breast Cancer Now. London; 2019. [Accessed 28th October, 2019]
3. Basu S, Rossary A, Vasson M-P. Role of inflammation and eicosanoids in breast cancer. Lipid Technology. 2016;28(3-4):60-64.
4. Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med. 2017;4:1673–11.
5. Leoncikas V, Wu H, Ward LT, Kierzek AM, Plant NJ. Generation of 2,000 breast cancer metabolic landscapes reveals a poor prognosis group with active serotonin production. Sci Rep. 2016;6:19771.
6. McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats Relationship with central serotonin turnover. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;83(4-6):185-191.
7. He Q, Shkarin P, Hooley RJ, Lannin DR, Weinreb JC, Bossuyt VIJ. In vivo MR spectroscopic imaging of polyunsaturated fatty acids (PUFA) in healthy and cancerous breast tissues by selective multiple-quantum coherence transfer (Sel-MQC): A preliminary study. Magn Reson Med. 2007;58(6):1079-1085.
8. Thomassin-Naggara I, Trop I, Lalonde L, David J, Péloquin L, Chopier J. Tips and techniques in breast MRI. Diagnostic and Interventional Imaging. 2012;93(11):828-839.
9. Bottomley PA. Spatial Localization in NMR Spectroscopy. Ann N Y Acad Sci. 1987;508(11):333-348.
10. Baik H-M, Su M-Y, Yu H, Nalcioglu O, Mehta R. Quantification of choline-containing compounds in Malignant breast tumors by 1H MR spectroscopy using water as an Internal Reference at 1.5 T. MAGMA. 2006;19(2):96-104.
11. Vanhamme L, van den Boogaart A. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35-43.
12. Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31(4):269-286.
13. Lundbom J, Hakkarainen A, Fielding B, et al. Characterizing human adipose tissue lipids by long echo time 1H-MRS in vivo at 1.5 Tesla: validation by gas chromatography. NMR Biomed. 2010;23(5):466-472.
14. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-410.
15. Tuominen VJ, Ruotoistenmäki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010;12(4):R56.
16. McClelland RA, Finlay P, Walker KJ, et al. Automated Quantitation of Immunocytochemically Localized Estrogen Receptors in Human Breast Cancer. Cancer Research. 1990;50(12):3545-3550.
17. Azrad M. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Frontiers in Oncology. 2013;3(224):1-12.
18. Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA. 1990;87(6):2187-2191.
19. Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology. 2005;180(1):12-20.
20. Ito T, Ikeda U, Shimpo M, Yamamoto K, Shimada K. Serotonin Increases Interleukin-6 Synthesis in Human Vascular Smooth Muscle Cells. Circulation. 2000;102(20):2522-2527.
Figures
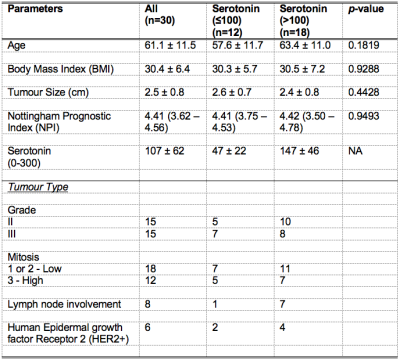
Table 1. Patient demography.
Patient demography and clinical histopathological findings of excised breast tumours are shown for each group and the entire cohort. Quantitative data are expressed as mean and standard deviation (apart from Nottingham Prognostic Index where median and interquartile range are shown), while qualitative data expressed as number of positive cases.
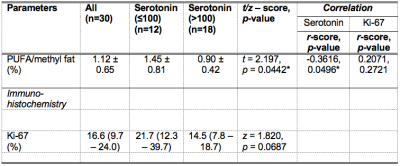
Table 2. Polyunsaturated fatty acids (PUFA) in whole breast tumours and histological markers serotonin and Ki-67.
Polyunsaturated fatty acids (PUFA) and tumour proliferative marker Ki-67 are shown for groups and the entire cohort. The spread of Ki-67 expression is non-normal and is reported as median and interquartile range (IQR). Correlation scores of PUFA against serotonin and Ki-67 are also shown. Significant findings are marked by ‘*’.
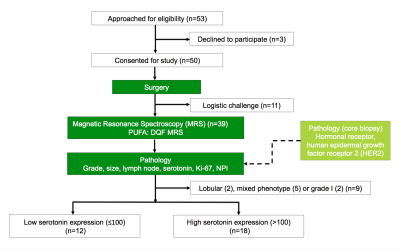
Figure 1. Study design.
The study adopted a two-group cross sectional arrangement as shown in this flow chart. Immediately after surgery, the freshly excised tumours were scanned on a clinical 3.0 T MRI scanner to derive polyunsaturated fatty acids (PUFA) using double quantum filtered (DQF) magnetic resonance spectroscopy (MRS). Subsequently, histopathological analysis provided serotonin, Ki-67 and Nottingham Prognostic Index (NPI). Thirty patients with invasive ductal carcinoma (IDC) were eventually entered into the study and used for all statistical analysis.
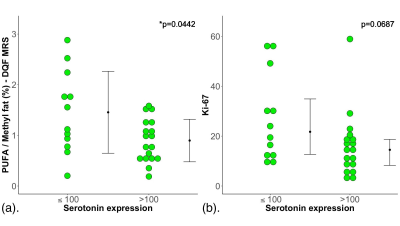
Figure 2. Group difference results.
The group difference in (a) polyunsaturated fatty acids (PUFA)/methyl fat (%) and (b) Ki-67 are shown in dot plots. Each dot represents the measurement obtained in each patient, and the dots are organised in two columns corresponding to low serotonin and high serotonin cases. For PUFA, the error bar indicates the mean and standard deviation. For Ki-67, it indicates the median and interquartile range. The t-test was performed between the groups for PUFA while Mann-Whitney U test was used for Ki-67. Statistically significant p values are marked by ‘*’.
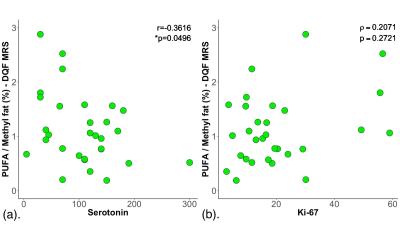
Figure 3. Correlation results.
Polyunsaturated fatty acids (PUFA)/methyl fat (%) was correlated against (a) serotonin expression and (b) Ki-67 expression within the entire cohort, and shown as scatter plots. The corresponding Pearson’s r (in (a)) and Spearman’s rank correlation rho (ρ) (in (b)) scores and p values are displayed. A significant negative correlation is observed between PUFA and serotonin expression. However, there is no significant correlation between PUFA and Ki-67 expression.