4804
Evaluate the Feasibility of Delayed Contrast Extravasation MRI to Delineate Sub-volume Target in brain tumors Radiotherapy.1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
Delayed contrast extravasation MRI (DCEM) could could differentiate regions of contrast agent clearance as an active tumor from regions of contrast agent accumulation as non-tumor tissues. By comparing the sub-volume of active tumor and non-tumor from DCEM with non-liquefaction necrosis and liquefaction from T2WI, our results showed that compared to liquefaction necrosis regions of the T2WI, the DCEM has advantages in distinguishing liquefaction area and could clearly differentiate sub-volume of active tumor from non-liquefaction. The application of DCEM was thus feasibly to guide the delineation of sub-volume target in brain tumor.
Introduction
The failure of radiotherapy is caused by the uneven distribution of tumor cells within a malignant brain tumor. Previous studies have found that the analysis of contrast clearance difference could provide a reliable assessment of tumor burden; however, no studies have applied it for radiation therapy. The main goal of this study was thus to evaluate the feasibility of delayed contrast extravasation MRI (DCEM) in differentiation of regions of contrast agent clearance as an active tumor from regions of contrast agent accumulation as non-tumor tissues so as to delineate sub-volume target in brain tumor radiotherapy.Methods
Subjects:Twenty-six patients (mean age: 57 ± 12 years old) with malignant brain tumor were scanned with MRI. The first and second acquisitions of standard T1-weighted images (T1WI) and T2-weighted images (T2WI) were respectively performed at 5 minutes and 60 minutes after injection of contrast agent.
MRI experiments:
All MRI experiments were performed at 3T MR system (Discovery 750w, GE Healthcare, USA) with brain coil. For the T1WI measurement, the scan parameters were applied as follows: TR = 8.47 ms, TE = 3.25 ms, matrix = 256 × 256, FOV = 256 × 256 mm, slice thickness = 1 mm. For the T2WI measurement, the scan parameters were applied as follows: TR = 13312 ms, TE = 113.5 ms, matrix = 416 × 416, FOV = 260 × 260 mm, slice thickness = 3 mm. A standard single dose (0.1 mmol/kg) of gadolinium DOTA was injected intravenously, total scan time was 12 mins10 seconds.
Data analysis:
The first acquired T1WI images were subtracted from the second ones for Contrast Clearance Analysis by using Brainlab software. DCEM computed by Brainlab concludes regions of contrast agent clearance which represent active tumor,and regions of contrast accumulation which represent non-tumor tissues. Based on T2WI images, 14 patients were divided into group A and group B, with and without liquefaction necrosis, respectively. Then, gross target volume (GTV) was delineated on T1WI images. Based on the GTV, active tumor and non-tumor regions were delineated on T1WI-DCEM fusion images, while liquefaction necrosis and non-liquefaction were delineated on T1-T2WI fusion images. Finally, the sub-volume of active tumor and non-tumor were compared respectively with the sub-volume of non-liquefaction and liquefaction necrosis on group A; the sub-volume of active tumor was compared respectively with the sub-volume of non-tumor on group B.
Paired t-tests was used to determine the difference of sub-volume between DCEM and T2WI. Significance threshold was set as p<0.05.
Results
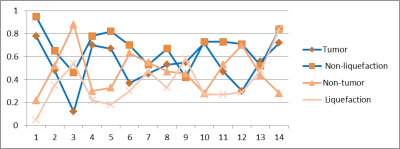
The mean value of GTV on enhanced T1WI in 26 patients was 10.28 ± 17.25 cm3.In group A, the mean value of GTVA on enhanced T1WI was 21.38 ± 25.70 cm3 . The non-liquefaction and liquefaction necrosis on T2WI were 13.65 ± 18.15cm3 and6.30 ± 7.57cm3, respectively. Active tumor area was 10.40 ± 13.52 cm3 while the non-tumor area was 9.55 ± 14.57 cm3, The non-liquefaction necrosis volume increased by an average of 28.2%(p<0.05 ; Fig.1), compared to active tumor area . While the non-tumor tissues increased by an average of 46.3% (p<0.05), compared to the liquefaction necrosis tissues .
In group B, the mean value of GTVB on enhanced T1WI was 4.39 ± 3.75 cm3. Active tumor area was 3.02 ± 2.78cm3 while the non-tumor area was 1.37 ± 1.42 cm3,The non-tumor tissues volume reduced by an average of 50.3% (p<0.05) , compared to the tumor activity area.
Discussion
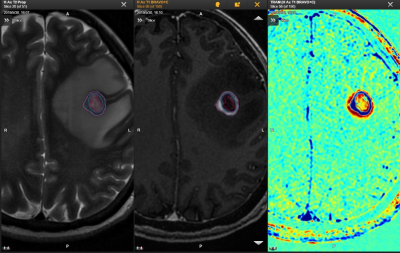
Heterogeneous distribution of tumor cells in GTV is a significant factor of radiotherapy failure,1 suggesting that sub-volume region of tumor activity may influence a radiotherapy plan. Consistent with previous studies,2,3 DCEM showed that morphologically active tumor could clearly differentiate tumor from non-tumor tissues. It could detect the liquefaction necrosis area and the incomplete liquefaction necrosis areas that were not able to be found on T2WI images. Physicians can follow DCEM to delineate the sub-volume of the target regions, and precisely give a higher dose in contrast agent clearance region marked as blue (Fig.2) in order to reduce the risk of radiotherapy failure in brain tumor, and consequently lower the recurrence rate.Conclusion
In conclusion, compared to T2WI, the DCEM has advantages in identifying the liquefaction area and could clearly differentiate sub-volume of active tumor from non-liquefaction necrosis. Therefore, DCEM is meaningful in guiding the delineation of sub-volume in primary and metastatic brain tumors.Acknowledgements
This research was supported by the National Key Research and Development Program of China (2017YFC0113202)References
1. Grosu AL,Souvatzoglou M,Röper B,et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with headand neck cancer. Int J Radiat Oncol Biol Phys 2007;69:541-551.
2. Zach L,Guez D,Last D,et al. Delayed contrast extravasation MRI for depicting tumor and non-tumoral tissues in primary and metastatic brain tumors. PLoS One 2012;7:e52008.
3. Zach L,Guez D,Last D,et al. Delayed contrast extravasation MRI: a new paradigm in neuro-oncology. Neuro Oncol 2015;17:457-465.
Figures