4802
DCE-MRI for detection of leukaemia induced bone marrow vascular permeability1Francis Crick Institute, London, United Kingdom
Synopsis
Acute Myeloid Leukemia (AML) is the most common acute leukemia in adults. While the clinical presentation is quite uniform, it is a highly heterogeneous disease at the genetic level. Using intravital two-photon microscopy, we have previously showed that AML patient-derived samples belonging to different genetic subgroups induced a common pathologic bone marrow (BM) vascular phenotype. To understand the translational potential of our findings we have optimized DCE-MRI for the assessment of bone marrow vascular permeability upon leukaemia development.
INTRODUCTION
Acute Myeloid Leukemia (AML) is the most common acute leukemia in adults. While the clinical presentation is quite uniform, it is a highly heterogeneous disease at the genetic level. Mouse models of AML are extensively used to better understand the pathobiology of the disease, to test potential novel therapies, and for the development of diagnostic and prognostic imaging tools [1-3]. Using intravital two-photon microscopy, we have previously showed that AML patient-derived samples belonging to different genetic subgroups induced a common pathologic bone marrow (BM) vascular phenotype [4]. To understand the translational potential of our findings we have optimized DCE-MRI for the assessment of bone marrow vascular permeability upon leukemia.METHODS
HL60, U937 and ML1 cell lines were grown in RPMI1640, and were tested for mycoplasma prior to commencing experiments. Media were supplemented with 10% FBS and 1x Penicillin-Streptomycin and all reagents were from Gibco-Life Technologies (Paisley, UK). Human AML patient samples (n=4) were obtained after informed consent at St Bartholomew’s Hospital (London, UK).All animal experiments were performed under the project license (PPL 70/8904) approved by the Home Office of UK and in accordance to The Francis Crick Institute animal ethics committee guidelines. NSG mice were injected with 2.0x106 HL60 cells in the tail vein. Two to three weeks after injection, MRI was performed followed by BM aspiration and flow cytometry to monitor the engraftment. T-cell depleted human AML cells (2.0x106 cells per mouse) were injected in NSG mice. Engraftment was assessed at 10 weeks from the injection. Once hCD45% in the BM was above 50%, mice underwent MRI followed by AraC (cytosine arabinoside) treatment (10 mg/kg/day) for 7 days. MRI and BM puncture were repeated one week after treatment cessation. Mice were classified as responders or not responders if after treatment the hCD45% in the BM was below or above 30% of the starting value respectively. Non-injected NSG mice were used as controls.
MRI was performed on a 9.4T horizontal bore system (Bruker GMBH) equipped with a B-GA12SH gradient coil system. RF transmission and reception was performed with a 40mm ID quadrature birdcage coil (Bruker GMBH). A series of Fast Low Angle Shot (FLASH) scans were used for femur localization and for slice positioning. DCE scans were performed using a FLASH with the following parameters: TR = 17.639ms; TE = 1.859 ms; FA = 10° ; Repetition = 1100; FOV 30x30x0.5mm3; matrix 128x128, and resolution of 234µm. Dotarem (0.4mL/Kg) was injected 4 mins after the start of the scan. Total scan duration was 41 mins. All mice were placed in a head-first prone position for imaging. Anaesthesia was induced and maintained using isoflurane (1–4%) in room air supplemented with oxygen (80%/20%). Temperature and respiration rate were monitored using SA Instruments system.
Non-model based parameters were quantified by drawing regions of interest over the bone marrow and muscle, and using in-house developed Matlab scripts (MathWorks; Natick, MA).
RESULTS
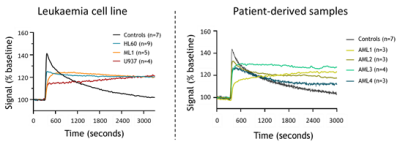
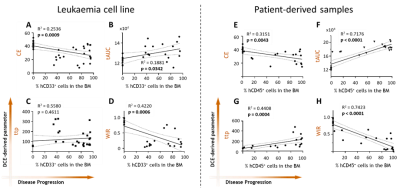
We first measured DCE-derived vascular parameters in control versus leukemic animals using different leukaemia cell lines. There is a clear difference in the BM kinetics in the presence of leukaemia, and it follows leukaemia progression (Fig.1). Also, changes in DCE-related parameters that are linked to poor vascular function are found to be correlated with disease progression (Fig.2A-D). This is also seen in patient-derived leukemic mice (Fig.2E-H).DISCUSSION / CONCLUSION
Our DCE-MRI results are in agreement with our previous result using intra-vital microscopy [4]. Our results further suggest that the detection of a pathologic vascular phenotype in the BM of AML patients could be of used to response to treatment and clinical outcome.Acknowledgements
The authors would like to thank Veronique Birault and members of the Translation team, Biological Research Facility, Flow Cytometry and In Vivo Imaging core facilities at the Francis Crick Institute for their valuable help. The authors are grateful to Prof. John Gribben (Barts) for providing human AML samples. A.L.G. was supported by an i2i translational grant scheme from the Francis Crick Institute. D.P. was supported by a non-clinical junior research fellowship from EHA. This work was supported by The Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001045), The UK Medical Research Council (FC001045), and the Welcome Trust (FC001045).References
1. Cook GJ, Pardee TS. Animal models of leukemia: any closer to the real thing? Cancer Metastasis Rev. 2013;32(1-2):63-76. Epub 2012/10/20. doi: 10.1007/s10555-012-9405-5. PubMed PMID: 23081702; PubMed Central PMCID: PMCPMC3568447.
2. Kohnken R, Porcu P, Mishra A. Overview of the Use of Murine Models in Leukemia and Lymphoma Research. Front Oncol. 2017;7:22. Epub 2017/03/08. doi: 10.3389/fonc.2017.00022. PubMed PMID: 28265553; PubMed Central PMCID: PMCPMC5317199.
3. Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23(7):877-89. Epub 2009/04/03. doi: 10.1101/gad.1771409. PubMed PMID: 19339691; PubMed Central PMCID: PMCPMC2666344.
4. Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell. 2017;32(3):324-41 e6. Epub 2017/09/06. doi: 10.1016/j.ccell.2017.08.001. PubMed PMID: 28870739; PubMed Central PMCID: PMCPMC5598545.
Figures