4787
Validation of SSIFT MRI: Differentiation between brain tumor and radiation necrosis1Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Chemical and Physical Biology Program, Vanderbilt University, Nashville, TN, United States, 3Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 4Department of Radiation Oncology, Vanderbilt University Medical Center, Nashville, TN, United States, 5Mallinckrodt Institute of Radiology, Washington University, St. Louis, MO, United States, 6Alvin J Siteman Cancer Center, Washington University, St. Louis, MO, United States
Synopsis
SSIFT is a recently developed MRI method that provides a specific means to detect brain tumors based on their differences in cell size. However, to date SSIFT has not been adequately validated. We therefore used computer simulations and studies of cultured cells in vitro and animal models in vivo to comprehensively validate SSIFT for brain cancer imaging. The results suggest SSIFT is highly specific for cell size and can differentiate brain tumors from other brain abnormalities such as peri-tumor edema and radiation necrosis, which cannot be reliably distinguished by other current MRI methods.
Introduction
Tumor recurrence is a common problem for brain cancer patients, and follow-up MRI scans are critical for its early detection. Unfortunately, recurrent tumor and radiation necrosis show similar signal abnormalities on standard-of-care MRI1. There is therefore still a need to develop an MRI method that can reliably differentiate these malignancies. SSIFT (selective size imaging using filters via diffusion times) is a recently developed method that provides a novel, specific means to detect brain tumors based on differences in cell sizes, and may address this challenge2. However, despite previous demonstrations of its application in brain cancer patients, SSIFT has not been comprehensively validated. We performed computer simulations and experimental studies of cultured cells in vitro, and animal tumor models in vivo to comprehensively validate the specificity of SSIFT for cancer cell sizes and the ability of SSIFT to distinguish primary glioma from radionecrotic lesions.Theory of SSIFT
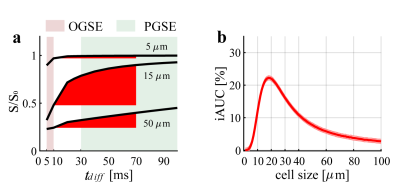
Diffusion-weighted MRI (DWI) signals are strongly dependent on the restriction (cell) size of biological tissues and the diffusion times used in acquisitions. With an appropriate choice of diffusion times and all other experimental parameters fixed, SSIFT defines a parameter iAUC (incremental area under curve) that emphasizes signals arising from specific restriction sizes (e.g. typical cancer cell size 15 – 25 μm) with simultaneous suppression of smaller (e.g. most brain cells < 10 μm) and larger (e.g. free water in edema or cysts) sizes. Figure 1 shows the definition of iAUC and how it serves as a filter to emphasize signals from cancer cells. Because cell sizes usually do not vary significantly in peri-tumor edema or radiation necrosis, SSIFT provides a unique means to differentiate tumors from other brain abnormalities.Methods
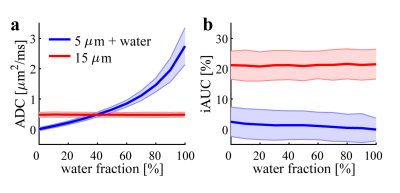
The acquisition protocol of SSIFT used on clinical 3T scanners was replicated in preclinical validation studies to ensure direct clinical relevance. Specifically, two diffusion times were used: 10ms (achieved with 1-cycle trapezoidal apodized cosine oscillating gradients with δ/∆ = 40/46 ms) and 70ms (using conventional trapezoidal pulsed gradients δ/∆ = 12/74 ms) with b = 1,000 s/mm2 and 32 directions.Computer simulations: Two tissues were simulated: (1) tumors modeled as packed spherical cells with a cell size of 15 μm; and (2) abnormal brain tissues modeled as packed smaller (5 μm) cells with varying fractions of free water. iAUC and ADC (apparent diffusion coefficient) were calculated and compared at a realistic signal-to-noise ratio of 20.
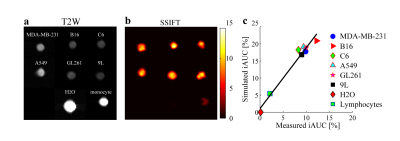
Cultured cells in vitro: Three primary brain cancer cell lines (9L, C6, and GL261) and three other cancer cell lines (A549, B16, and MDA-MB-231) were cultured and spun down as cell pellets for SSIFT imaging. The latter three cell lines represent three common metastatic brain cancers, i.e. lung cancer, melanoma, and breast cancer, respectively. For comparison, doped water and lymphocytes were also imaged. Ground truth cell sizes were estimated using light microscopy and then used to calculate the theoretical iAUC values. Values derived from SSIFT imaging and theoretical iAUC values were compared to evaluate the specificity of SSIFT to cell size.
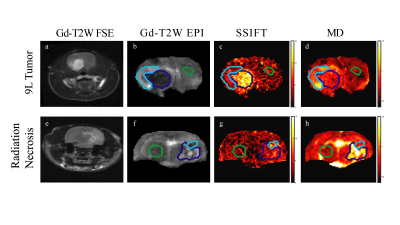
Animal models in vivo: 9L gliosarcoma tumors were induced in Fisher 344 rats by injecting 9L cells, and radiation necrosis was induced using a clinical linear accelerator. All images were acquired on a 4.7T Varian/Agilent MRI system. Clinical standard Gd-enhanced images were also acquired for comparison.
Results
Figure 2 shows the extent to which simulated ADC and iAUC values for different water contents distinguish tumors from brain abnormalities. The results of Figure 2a are consistent with previous findings that ADC has only moderate diagnostic accuracy in identifying glioma from radiation necrosis3. By contrast, iAUC can distinguish tumor from brain abnormalities independent of water fraction, indicating SSIFT is able to reliably detect brain tumors.Figure 3 demonstrates the dependence of SSIFT iAUC on cell sizes in cultured cells in vitro. Clearly iAUC is sensitive to large cancer cells and insensitive to small cells (e.g. lymphocytes) and free water. Moreover, the predicted iAUC correlates strongly with those obtained using SSIFT imaging (r = 0.98, p < 0.01 using Pearson correlation).
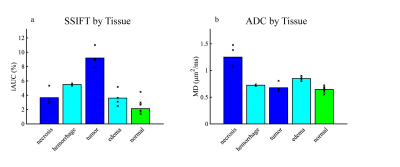
Figure 4 shows representative images of animals with a 9L tumor and radiation necrosis, and Figure 5 compares the capability of iAUC and ADC to differentiate tumors from brain abnormalities in 8 animals. iAUC shows high specificity for the tumor ROI and can distinguish tumor from other brain abnormalities and normal appearing tissues. Although ADC shows some ability to differentiate tumor from radiation necrosis, it shows lower contrast to noise ratio (CNR) than iAUC and cannot distinguish tumors from e.g. edema or hemorrhage within lesions, diminishing diagnostic potential.
Discussion and Conclusion
Despite prior applications of SSIFT imaging in metastatic brain cancer patients, SSIFT has not been validated previously. Here we validated that SSIFT is highly specific to large cancer cells and immune to the influences of free water in edema and necrosis. This enables SSIFT to reliably differentiate tumors from other brain abnormalities. SSIFT is readily achievable on clinical 3T scanners with a short acquisition time (<10 mins) so it is suitable to be included as an “add-on” to standard-of-care protocols whenever there is a clinical need to distinguish brain tumors from other abnormalities, especially radiation necrosis.Acknowledgements
No acknowledgement found.References
1. J. D. Rubenet al., Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. International journal of radiation oncology, biology, physics 65, 499-508 (2006).
2. J. Xuet al., in Proceedings of the 26th Annual Meeting of ISMRM. (Paris, France, 2018), pp. 1615.
3. H. Zhang, L. Ma, C. Shu, Y. B. Wang, L. Q. Dong, Diagnostic accuracy of diffusion MRI with quantitative ADC measurements in differentiating glioma recurrence from radiation necrosis. Journal of the neurological sciences 351, 65-71 (2015).
Figures