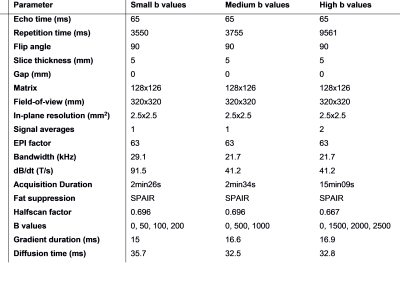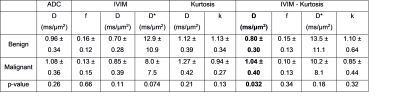4781
Comparison of ADC, IVIM and Kurtosis for differentiation of mesorectal lymph nodes in rectal cancer1Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal, 2University College London, London, United Kingdom, 3Champalimaud Clinical Centre, Champalimaud Centre for the Unknown, Lisbon, Portugal, 4Nova Medical School, Lisbon, Portugal, 5Philips Healthcare Iberia, Madrid, Spain
Synopsis
In this study we employed a clinically feasible diffusion MRI acquisition and modelling approach at 1.5T to characterize perfusion (IVIM) and higher order diffusion (Kurtosis) properties of mesorectal lymph nodes in rectal cancer patients upon staging. The results showed that diffusivity estimated from the IVIM-Kurtosis model was the only parameter showing significant differences between benign and malignant lymph nodes. Moreover, ROC analysis evidenced improved differentiation when adding IVIM-Kurtosis to standard T2-weighted qualitative assessment by expert radiologists.
Introduction
Lymph Node (LN) staging is one of the main determinants of the management of rectal cancer patients, yet current imaging methods show limited accuracy for that purpose1. Diffusion MRI (dMRI) is becoming an increasingly important tool for non-invasive detection of malignant lymph nodes2-4, with IVIM5,6 and Kurtosis7 models showing improved results compared to the standard ADC. Combining IVIM and Kurtosis could further improve LN differentiation8, however, to our knowledge, it has not been employed so far in mesorectal LN.This work employs a multi b-value clinical dMRI acquisition at 1.5T and investigates the ability of ADC, IVIM, Kurtosis and IVIM-Kurtosis approaches to differentiate between benign and malignant lymph nodes in patients with rectal cancer. We further compare the performance of dMRI models to standard T2-weighted qualitative assessment by expert radiologists.
Methods
Institutional setting, approved by ethics committee: A total of 10 patients previously diagnosed with rectal cancer (mean age of 64.9 years, 5 males) were enrolled, after obtaining written informed consent. The imaging was performed on a 1.5T Philips scanner with the diffusion acquisition added to the clinical staging pelvic MRI.Image acquisition: The diffusion data was acquired with multi-shot Spin-echo Echo-planar Imaging (SE-EPI), with the acquisition parameters detailed in Table 1. The diffusion acquisition was split in three parts, with small b-values (50, 100, 200 s/mm2) medium b-values (500, 1000 s/mm2) and high b-values (1500, 2000, 2500 s/mm2), to minimize the echo time of the acquisition (TE = 65 ms). In total, 76 lymph nodes were delineated by a radiologist (8 years experience) and matched to pathology during macroscopy. Due to motion artifacts, 4 nodes were excluded, leaving 72 nodes (14 malignant).
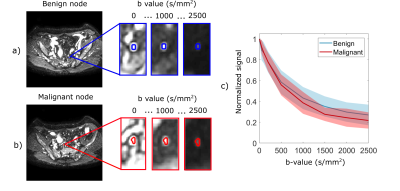
Data analysis: Whole-node ROIs were defined on the high b-value images and copied to the low and medium b-value data sets (Figure 1), and slightly translated to account for motion when necessary. The average ROI signal for each b-value was normalized to the ROI average of the b0 images in the respective set. The normalized signal decay was fitted with four diffusion models: ADC was fitted to the measurements with medium b-values, IVIM was fitted to the measurements with small and medium b-values, Kurtosis to the sets with medium and high b-values and IVIM-Kurtosis to the entire data set.
To investigate the parameter differences between benign and malignant nodes, we employed a mixed effect linear model with the diffusion metrics as response variable, and the malignancy status as predictor variable, with LNs grouped by patient. To assess the differentiation ability of different models, we employed a ROC analysis.
Results
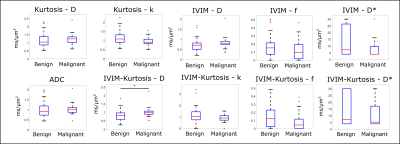
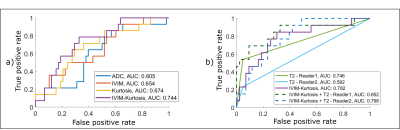
The normalized signal decay in Figure 1c clearly revealed a faster decay for malignant nodes and a slower decay for benign nodes, which are also reflected in the model parameters presented in Figure 2a and Table 2. Figure 2 shows box-plots of the parameter values for ADC, IVIM, Kurtosis and IVIM-Kurtosis estimated in benign and malignant nodes. ADC values were higher in malignant nodes compared with benign nodes; however, the difference was not statistically significant (P=0.26). The only parameter which reached a statistically significant threshold was the diffusivity (D) estimated from the IVIM-Kurtosis model (P=0.032), which has higher values in the malignant nodes.Figure 3b presents the ROC analysis which compared the four diffusion models in terms of differentiating between benign and malignant lymph nodes. ADC is the worst performing model, with an area-under-the-curve (AUC) of 0.60, while IVIM-Kurtosis is the best performing one with an AUC of 0.74, however, the difference does not reach statistical significance according to the DeLong test (P=0.16). Figure 3c shows that IVIM-Kurtosis also provides a better differentiation between benign and malignant nodes (AUC=0.78) compared to the standard clinical T2 classification based on the ESGAR 2016 criteria (57) (AUC=0.74 for Reader 1 and AUC=0.59 for Reader 2), which takes into consideration lymph node size, shape, contour and heterogeneity. Moreover, combining IVIM-Kurtosis and T2 results significantly improves the classification compared to T2 weighted images alone (P=0.08 for Reader 1 and P=0.007 for Reader 2).
Discussion
The current study shows that accounting for IVIM and Kurtosis effects provides better differentiation between benign and malignant mesorectal lymph nodes compared to standard ADC and has the potential to improve LN classification when considered alongside T2 weighted images. One limitation of this study is the relatively small number of malignant lymph nodes (14/72) and the fact that half of them originate from only one patient. Nevertheless, this effect was accounted for in the statistical model. In this study we focused on a ROI analysis to mitigate the effects of motion, nevertheless in future studies we will also attempt to perform image registration to enable voxelwise parameter estimation.Conclusion
Quantitative IVIM-Kurtosis modelling of dMRI data shows potential to improve the differentiation of benign and malignant mesorectal lymph nodes.Acknowledgements
This study was funded by the Champalimaud Centre for the Unknown. Dr. Andrada Ianus´ and Prof. Daniel C. Alexander’s work was also supported by EPSRC grants EP/M020533/1 and EP/N018702/1 and the NIHR UCLH Biomedical Research Centre. The contributions of Paula Montesino, Nuno Loução and Javier Gonzales-Sanchez were funded by Philips Healthcare Iberia.References
[1] Grone, J. et al, Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer with Magnetic Resonance Imaging, J Gastrointest Surg (2018) 22: 146.
[2] Ogawa, M., et al., The Usefulness of Diffusion MRI in Detection of Lymph Node Metastases of Colorectal Cancer. Anticancer Research, 2016. 36(2): p. 815-9.
[3] Yasui, O., M. Sato, and A. Kamada, Diffusion-weighted imaging in the detection of lymph node metastasis in colorectal cancer. Tohoku J Exp Med, 2009. 218(3): p. 177-83.
[4] Heijnen, L.A., et al., Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol, 2013. 23(12): p. 3354-60.
[5] Qiu L, et al. Role of quantitative intravoxel incoherent motion parameters in the preoperative diagnosis of nodal metastasis in patients with rectal carcinoma. J. Magn. Reson. Imaging. 2016;44: 1031-9.
[6] Yu XP, et al. Discrimination between Metastatic and Nonmetastatic Mesorectal Lymph Nodes in Rectal Cancer Using Intravoxel Incoherent Motion Diffusion-weighted Magnetic Resonance Imaging. Acad Radiol. 2016;23:479–85.
[7] Yu, J., et al. Diffusion kurtosis imaging in identifying the malignancy of lymph nodes during the primary staging of rectal cancer. Colorectal Dis. 2018;20(2):116-25.
[8] Lu, Y. et al. Extension of the intravoxel incoherent motion model to non‐gaussian diffusion in head and neck cancer, J. Magn. Reson. Imaging 2012;36:1088–1096.
Figures