4770
Controlling Apparent Diffusion and Kurtosis with Vesicle Size in Quantitative Diffusion Kurtosis Phantoms1Department of Radiology, University of Michigan, Ann Arbor, MI, United States, 2Department of Chemistry, University of Michigan, Ann Arbor, MI, United States
Synopsis
Chemical composition of lamellar lipid vesicles determines particle diameter and hence the size of a restricted diffusion component. Phantoms made at 1% by weight solid using cetearyl alcohol and cetyl trimethyl ammonium bromide with varying molar ratios have different apparent diffusion constants and kurtosis values. Vesicle sizes are confirmed by dynamic light scattering and electron microscopy. These molecular vesicles provide a basis for a quantitative diffusion phantom and future MR studies of nanoscopic domains.
Introduction
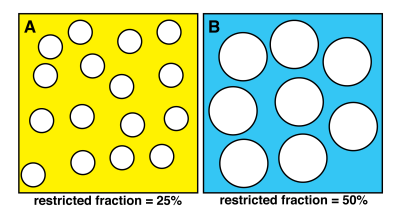
Phantoms with known MR parameters are needed to validate quantitative measurements in multisite MRI studies (1). Our previous work shows that changing the concentration of lamellar lipid vesicles creates diffusion phantoms with tunable apparent diffusion and kurtosis values, Dapp and Kapp (2). In this work, we vary the molar ratio of lipid to surfactant to change vesicle diameter and restricted diffusion fraction; consequently changing Dapp and Kapp values. It is easily shown that given a constant surface area, i.e. a constant weight fraction of molecules, that the volume enclosed by vesicles will increase linearly with vesicle diameter. Figure 1 provides the 2D analogy to the 3D case and helps to visualize how increasing diameter will increase restricted fractions of water molecules.Methods
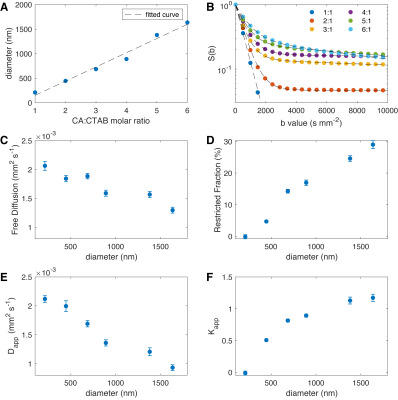
Vesicles were made at constant 1% (w/w) solids-to-water of cetearyl alcohol (CA) and cetyl trimethyl ammonium bromide (CTAB), with CA:CTAB molar ratio varying from 1:1 to 6:1. CTAB was dissolved in hot water (80 °C) and melted CA was added to form vesicles by stirring and cooling below the lipid transition temperature. All samples were filtered with through a 5 micron polycarbonate filter. Dynamic light scattering was performed at 1 mg/ml concentration and cumulant particle diameter, <d>, and poydispersity index, pi, were determined. Transmission electron microscopy (TEM) was performed on representative samples to visualize vesicle shape and dimensions. NMR water signals were recorded on an Agilent 700 MHz system using a dual stimulated echo experiment to suppress convective flow with b values up to 10,000 s/mm2. Diffusion time Δ was 100 ms and gradient duration δ was 2 ms. The diffusion NMR signal was fitted in Matlab to a biexepnential model with free and restricted diffusion, and to a kurtosis model with apparent diffusion and kurtosis values at appropriate b values (1,2). The biexponential model is given as $$$S(b)=p_{free} exp(-b D_{free}) +p_{rest.} exp(-b D_{rest.})$$$ and the kurtosis model is given as $$$ln(\frac{S(b)}{S(0)})=-b D_{app}+1/6 K_{app}(b D_{app})^2$$$ (1,2). Fitted results are shown in Fig. 3(C,D) for the biexponential model and Fig. 3(E,F) for the kurtosis model with error bars representing 95% CI.Results
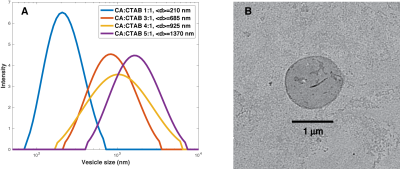
The ratio of the lipid to surfactant determines vesicle diameter in a controlled fashion. The diameter of vesicles as measured by DLS varies from 210 nm up to 1,380 nm (Fig. 2A). Representative vesicles are seen as spherical by TEM (Fig. 2B). Vesicle diameter is proportional to CA:CTAB ratio (Fig. 3A) and a biexponential model of diffusion with free and restricted components fits all data well (Fig. 3B). Free diffusion, Dfree, decreases from about 0.002 to 0.0015 mm2/s in the biexponential model (Fig. 3C) whereas apparent diffusion Dapp changes from 0.0021 to about 0.0009 mm2/s, highlighting model differences. More importantly, the restricted fraction in the biexponental model is proportional to the vesicle diameter, as predicted by conservation of mass and geometry (Fig. 3D). Kurtosis value (Kapp) changes from 0 for the 210 nm vesicles to 1.17 for the 1,640 nm vesicles (Fig. 3F).Discussion
The ability to choose vesicle diameter imparts increased control of phantom diffusion properties and allows specific values of Dapp and Kapp in a quantitative diffusion phantom. In addition, samples with vesicles of known diameter provide opportunities to study novel diffusion MRI experiments tuned to a particular vesicle size or microenvironment (3). These vesicles have polydispersity indices between 0.2 and 0.3, with the larger vesicles becoming more disperse. Though not done here, polydispersity could be reduced further by passing vesicles through appropriate sized filters.These findings are useful because the 1% (w/w) samples have low viscosity, flow easily, and do not trap air or bubble as may happen in gels or samples of higher viscosity. In addition, starting from low viscosity and relatively large value of Dapp, it would be easy to reduce Dapp with a polymer such as PVP, yet leave the Kapp unaffected (4).
Conclusion
Lamellar vesicles provide an ideal molecular platform for quantitative diffusion phantoms. They are stable (1), easily generated, and can be made at large volumes with tunable diffusion parameters. Additional refinement of fabrication of these systems will provide increased utility to benchmark MRI systems in muticenter clinical trials and provide well characterized nanoscopic platforms for future development of novel MRI pulse sequences.Acknowledgements
National Institutes of Health Grants: U01CA166104, U24CA237683, P01CA085878 and P30 CA008748.References
1. Malyarenko DI, Swanson SD, Konar AS, et al. Multicenter Repeatability Study of a Novel Quantitative Diffusion Kurtosis Imaging Phantom. Tomography. 2019;5(1):36-43
2. Swanson SD, Malyarenko DI, Chenevert TL. Tunable diffusion kurtosis in lamellar vesiclesuspensions toward development of quantitative phantom surrogate of tumor microenvironment. Proc. Intl Soc Mag Reson Med 2019, TP3632, Montreal CA.10.
3. Le Bihan D. Mousion, Tissue microdynamics and microstructure. NMR Biomed. 1995;8(7):375-386.
4. Pierpaoli C, Sarlls J, Nevo U, Basser PJ, Horkay F, editors. Polyvinylpyrrolidone (PVP) water solutions as isotropic phantoms for diffusion MRI studies. Proc. Intl Soc Magn Reson Med; 2009; Honolulu HI.
Figures