4760
Value of Gd-EOB-DTPA-enhanced MRI in detecting residual hepatocellular carcinoma and evaluating regional hepatic function injury afterTACE1Third Affiliated Hospital of Soochow University, changzhou, China, 2First Hospital of Lanzhou University, lanzhou, China, 3Philips Healthcare, Shanghai, China, shanghai, China
Synopsis
In this study, we sought to investigate the value of Gd-EOB-DTPA-enhanced MRI in diagnosing residual hepatocellular carcinoma (HCC) and evaluating regional hepatic function injury after transarterial chemoembolization (TACE).Gd-EOB-DTPA-enhanced MRI is a sensitive imaging in diagnosing residual HCCs, especially for the imaging feature of arterial enhancement and DWI hyperintensity, and SNR value measured on HBP can be used as a valuable index to assess regional hepatic function injury after TACE.
Introduction
Transarterial chemoembolization (TACE) has been proved to be valuable in slowing tumor progression and improving survival prognosis in patients with unresectable hepatocellular carcinoma (HCC) (1). Unfortunately, despite hepatic function injury associated with TACE may limit the option of repeated treatment, residual HCCs can induce neovascularisation and continued growth, which may adversely affect the post-TACE recovery and worsen prognosis of patients. Therefore, accurate diagnosis of residual HCCs and assessment of regional hepatic function injury after TACE would be critical needed for determining the success of treatment and guiding subsequent therapeutic planning. Up to now, there are only fewer well-validated studies (2) that have been performed to explore the value of Gd-EOB-DTPA-enhanced MRI in diagnosing residual HCCs and evaluating regional hepatic function injury after TACE, and to the best of our knowledge, no studies have focused on the imaging features in identifying residual HCCs from necrotic tumors. Therefore, we conducted this retrospective study to investigate the value of Gd-EOB-DTPA-enhanced MRI in detecting residual HCCs, and to evaluate regional hepatic function injury after TACE.Material and Methods
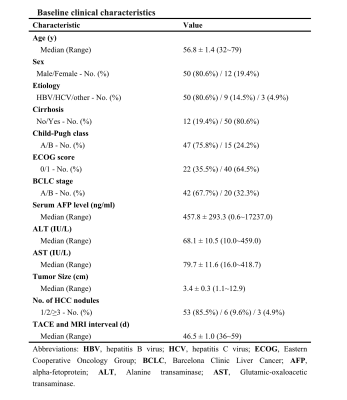
From June 2017 to May 2019, the clinical data of 62 consecutive patients with 74 diagnosed HCC who were scheduled for TACE therapy and a follow-up of Gd-EOB-DTPA-enhanced MRI at our hospital were retrospectively analyzed. The reference standard was: 1) pathological result suggesting HCC; 2) tumour enhancement or staining on DSA; 3) imaging method with at least 6 months follow-up, revealing arterial enhancement or tumor progession. Post-TACE imaging features of T1WI, T2WI, DWI, arterial-, portal venous-, delayed-, and HBP were classified as hyperintense, isointense and hypointense, homogeneous or inhomogeneous, respectively, compared to adjacent liver parenchyma. Diagnostic criteria of Gd-EOB-DTPA-enhanced MRI in detecting residual HCCs: arterial enhancement, venous washout or HBP hypointensity. Conversely, no enhancement was interpreted as necrotic tumor. Additionally, signal to noise ratio (SNR) were manually measured at peritumoral regions (within 3cm of lesion treated by TACE), nonperitumoral regions and backgound regions (outside of MRI image) in the T1WI image before and 20 min after Gd-EOB-DTPA adminstration.Results
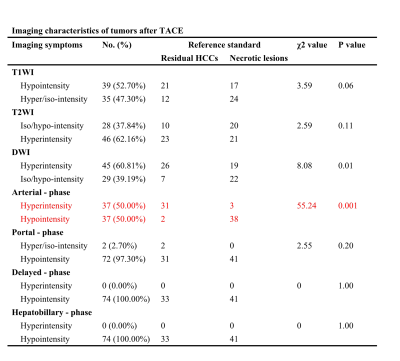
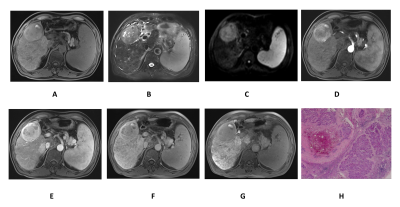
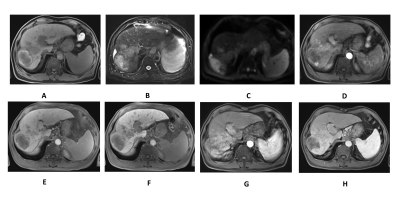
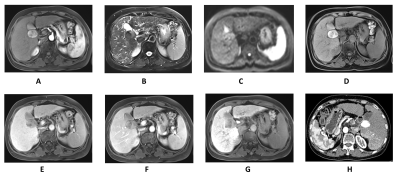
The baseline characteristics of enrolled patients are summarized in Fig.1. Fig.2 demonstrates the Gd-EOB-DTPA-enhanced MRI features of 33 residual HCCs and 41 necrotic tumors. Residual HCCs (Figure.1-2) presented the imaging characteristics of T1WI hypointensity (21/33), hyperintensity on T2WI (23/33) and DWI image (26/33), arterial enhancement and washout in portal venous phase were shown in 31 residual HCCs, all residual HCCs presented washout at delayed phase and HBP. Nevertheless, most necrotic tumors (Fig.3) displayed increased T1WI signal (24/41) without arterial enhancement (38/41); and all necrotic tumors presented washout at portal venous phase, delayed phase and HBP. Characteristics of DWI hyperintensity and arterial enhancement were of value in differentiating residual HCCs and necrotic tumors (χ2 = 0.01, 0.001, respectively, with P<0.05). No significant difference was found among the imaging intensity of T1WI, T2WI, portal venous phase, delayed phase and HBP. Therefore, the sensitivity and specificity of arterial enhancement in diagnosing residual HCCs were 93.9% and 92.7%, and they were 78.8% and 53.7% for DWI hyperintensity, respectively. The sensitivity, specificity, PPV, NPV and accuracy of Gd-EOB-DTPA-enhanced MRI in diagnosing residual HCCs were 93.9%, 85.4%, 83.8%, 94.6% and 89.19%, respectively. The SNR values of peritumoral region (540.70 ± 15.10) was signifcantly lower than that of nonperitumoral region (625.71 ± 18.28), when measured at HBP.Discussion
Colliquative or coagulative necrosis, intratumoral hemorrhage and inflammatory infiltration are accompanied with lipiodol deposition in HCC lesion following TACE, and all this pathological tissue can lead to hyperintensity and hypointensity in T1WI and T2WI image simultaneously[3], thus contributing to these imaging features demonsrate to be difficult in differentiating necrotic tumors.After TACE treatment, HCC tumor cells undergo necrosis and apoptosis with cell membrane permeability increased and cell gaps enlarged, and those lead to more water molecule movement and decreased signal on DWI [4]. While for residual HCCs, the tortuosity of extracellular space and hydrophobic cellular membranes will increase cell density and therefore restrict the diffusion of water protons, thus presenting increased signal on DWI.
The overwhelming majority of residual HCCs (31/33) were presented as rapidly enhanced portions at arterial phase, homogeneous or inhomogeneous, whereas the lack of arterial enhancement corresponded to existence of necrotic tumors. At the portal venous phase, most residual HCCs turned into hypointense, and just two residual HCCs continued to be persistent enhancement, indicating potential feeding artery from portal venous. Both residual HCCs and necrotic tumors demonstrated complete enhancement loss at the delayed phase, representing the characteristic contrast enhancement patterns of residual HCC was hypervascular (wash-in) at the arterial phase, followed by hypovascular appearance (wash-out) at the portal venous and delayed phase.
Chemotherapeutic drug perfusion and embolic agents introduced to hepatic artery are bound to cause peritumoral liver tissue injury induced by ischemia and hypoxia of hepatocytes [5]. Such injury could decrease the number of functional OATP1 transporter, and therefore the uptake of Gd-EOB-DTPA will be decreased to a great extent in the peritumoral region. In this study, no signifcant difference of SNR value was found between peritumoral regions and nonperitumoral liver tissue on unenhanced T1WI image. However, peritumoral region exhibited significantly lower Gd-EOB-DTPA uptake compared with nonperitumoral region, indicating regional hepatocyte injury caused by TACE therapy.
Acknowledgements
NAReferences
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA, 2018, 68(6):394-424.
2. Liu HF, Zou LQ, Lu XR, et al. Diagnostic Efficacy of Contrast-Enhanced MRI in Detecting Residual or Recurrent Hepatocellular Carcinoma After Transarterial Chemoembolization: A Systematic Review and Meta-analysis .J Magn Reson Imaging. 2019 Nov 1.
3. Liu R, Wang JH, Zhou KR, et al. A comparative study of MRI manifestations and pathological changes in hepatocellular carcinoma treated by transcatheter arterial chemoembolization with lipiodol. Chin J Hepatol, 2005, 13(10):754-758.
4. Mannelli L, Kim S, Hajdu CH, et al. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: Prediction and assessment of response to transarterial chemoembolization. Preliminary experience. Eur J Radiol, 2013, 82(4):577-582.
5. Lee S, Kim KM, Lee SJ, et al. Hepatic arterial damage after transarterial chemoembolization for the treatment of hepatocellular carcinoma: comparison of drug-eluting bead and conventional chemoembolization in a retrospective controlled study. Acta Radiol, 2017, 58(2):131-139.
Figures