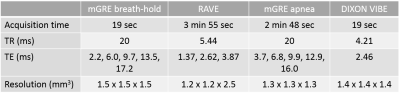
4759
USPIO-Enhanced Imaging of Mediastinal Lymph Nodes in Esophageal Cancer with Sparse Radial MRI1Department of Radiology and Nuclear Medicine, Radboud university medical center, Nijmegen, Netherlands, 2Department of Surgery, Radboud university medical center, Nijmegen, Netherlands, 3Department of Radiology, New York University, New York, NY, United States
Synopsis
Clinical imaging techniques are unable to outline metastatic spread of esophageal cancer to nearby lymph nodes. This deficiency is predominantly due to significant motion in the upper abdomen. We radially acquired MRI data with a contrast agent of USPIO nanoparticles to image the lymph nodes of an esophageal cancer patient. After retrospective gating the data into motion phases we created images with a compressed sensing reconstruction. We also acquired traditional multi-gradient echo sequences in breath-hold and apnea. The radially acquired, compressed sensing reconstructed and motion-gated images appear of similar diagnostic quality to those obtained with traditional modalities.
Introduction
Esophageal cancer (EC) is in the top ten of most common causes of cancer related deaths in both men and women in the Western world.1 Current treatment of resectable esophageal cancer consists of a combination of radiotherapy, chemotherapy and surgery. However, despite progress made in the last decades, the 5-year survival rate is estimated at only 10%.2 One important reason for this poor outcome is the lack of imaging tools to characterize early metastatic spread to the surrounding lymph nodes, which is needed to guide the choice for, and the extent of surgery. Ultra-small superparamagnetic iron oxide (USPIO) nanoparticles are used as a novel MRI contrast agent to detect metastases in lymph nodes with T2*-weighted MRI3, but cardiac and respiratory motion in this specific area complicate high resolution distortion-free imaging. We applied an undersampled golden angle radial stack-of stars MR sequence with three gradient echoes and compressed sensing reconstruction to create high resolution T2*-weighted images of a patient with esophageal cancer during continuous breathing, and assessed the feasibility of discriminating lymph nodes with and without USPIO uptake. We compare the results with diagnostic images from traditional sequences in breath-hold and apnea.Methods
Twenty-four hours after administration of USPIO nanoparticles (Ferumoxtran-10, SPL medical, Arnhem, the Netherlands), just prior to start of surgery, we acquired USPIO-enhanced MR data covering the thorax of a EC-patient under general anesthesia at 3 Tesla (Skyra, Siemens Healthineers, Erlangen). Data acquisition and evaluation was in accordance with the local ethics committee and informed consent was obtained. We acquired a transverse triple-echo gradient-echo (mGRE) golden angle radial stack-of-stars (RAVE) sequence. Respiration of the patient was induced by a pressure ventilator at a frequency of 20 cycles/min. Next to the RAVE sequence, also traditional 3D mGRE data was acquired, both in controlled breath-holds of 18 seconds as well as in prolonged apnea of 4 minutes. Sequence parameters for each of the different experiments are displayed in Table 1. Respiratory motion signals used for self-gating were estimated from the k-space centers of the acquired spokes before image reconstruction.4 To remove motion artifacts, we used the motion signal’s amplitude at each time point to bin the data set into eight equally spaced bins that represent the different respiratory phases. The undersampled k-space was transformed to image space by a compressed sensing (CS) algorithm with a total variation minimization along the temporal dimension.4 For each sequence, the root-sum-of-squares signals of individual echoes were used to increase T2*-weighting and signal-to-noise ratio (SNR). A radiologist categorized the lymph nodes in the images resulting from the traditional sequences as either suspicious or non-suspicious for metastases.Results
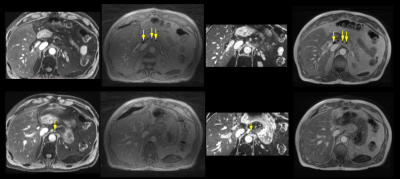
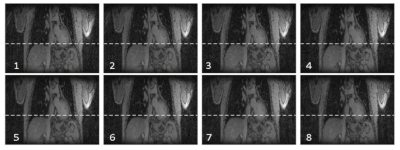
All acquired 3D datasets were of diagnostic quality (Fig. 1). Lymph nodes with USPIO-uptake had lost MR signal intensity and were visible as small black spheres within lipid tissue (arrows in top row Fig. 1). Visible lymph nodes on water-excited, T2*weighted scans have taken up little or no nanoparticles, and are suspicious for having metastases (arrows in bottom row Fig. 1). The in-phase Dixon images are displayed for anatomical clarity. Black lymph nodes are clearly visible in the CS reconstruction of the RAVE sequence, without requiring breath-hold and with the highest in-plane spatial resolution. The suspicious node was identified by its brightness on the water-excited T2*-weighted mGRE sequences. This node also retained signal intensity on the radial scan, although without lipid suppression or water excitation and due to the shorter echo times, the contrast is less pronounced for the latter. Figure 2 illustrates the successful motion detection and gating of the data into eight different motion phases. The phases show only minor respiratory motion as performed by the mechanical ventilator.Discussion
Our acquisition and reconstruction strategy shows promise for assessing USPIO-uptake in para-esophageal lymph nodes, which is of aid in identifying nodal metastases. Acquisition is performed in a non-invasive manner and reconstruction is successful in the presence of regular respiratory motion. Both SNR and image resolution are increased as data can be acquired continuously and is independent on patient breath-hold. Adjusting the pulse sequence to include more gradient echoes (with longer TEs) will allow the creation of computed TE-images which may show stronger image contrast for suspicious nodes, but this may take additional sequence adjustments to maintain sufficient temporal resolution for motion mitigation. A greater contrast level can also be achieved by applying a water-selective excitation pulse in future experiments.Conclusion
We proposed a method for detecting USPIO uptake in lymph nodes of a patient with esophageal cancer. Our method does not depend on breath-hold or apnea but can instead be applied during free breathing, thereby greatly reducing the physical stress on the patient. Our results hold promise for an increase in spatial resolution and SNR over the standard clinical methods.Acknowledgements
No acknowledgement found.References
1. F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, and A. Jemal, “Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA: A Cancer Journal for Clinicians, vol. 68, no. 6, pp. 394-424, 2018.
2. A.T. Gavin, S. Francisci, R. Foschi, D.W. Donnelly, V. Lemmens, H. Brenner, and L.A. Anderson, “Oesophageal cancer survival in Europe: A EUROCARE-4 study,” Cancer Epidemiology, vol. 36, no. 6, pp. 505-512, 2012.
3. M. Harisinghani, J. Barentsz, P. Hahn, W. Deserno, S. Tabatabaei, C. Van de Kaa, J. Dela Rosette, and R. Weissleder, “Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer,” New England Journal of Medicine, vol. 348, no. 25, pp. 2491–2499, 2003.
4. L.
Feng, L. Axel, H. Chandarana, K. T. Block, D. K. Sodickson, and R. Otazo,
“XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state
dimensions using com-pressed sensing,” Magnetic Resonance in Medicine, vol. 75,
no. 2, pp. 775–788, 2016.
Figures