Fuminori Hyodo1, Shinichi Shoda1, Tomoko Nakaji2, Hinako Eto3, Tatsuya Naganuma2, Norikazu Koyasu1, Masaharu Murata3, and Masayuki Matsuo1
1Gifu University, Gifu, Japan, 2Japan Redox Inc., Fukuoka, Japan, 3Kyushu University, Fukuoka, Japan
Synopsis
Malignant melanoma is one of the most progressive tumors in
humans with increasing incidence worldwide. Dynamic nuclear polarization
(DNP)-MRI is a noninvasive imaging method to obtain the spatio-temporal
information of free radicals. If endogenous free radicals in melanin pigment
could be utilized as a bio-probe for DNP-MRI, this will be an advantage for the
specific enhancement of melanoma tissues. We report that biological melanin
pigment induced a in vivo DNP effect by interacting with water molecules. In
addition, we demonstrated in vivo melanoma imaging based on the DNP effects of
endogenous free radicals in the melanin pigment of living mice.
Introduction
Melanin is a pigment that includes
free radicals and is widely distributed in living animals. Malignant melanoma
is one of the most progressive tumors in humans with increasing incidence
worldwide, and has shown resistance to chemotherapy, resulting in high mortality
at the metastatic stage. In general, melanoma involves the abnormal
accumulation of melanin pigment produced by malignant melanocytes. Electron
paramagnetic resonance (EPR) spectroscopy and imaging is a powerful technique
to directly visualize melanomas using endogenous free radicals in the melanin
pigment. Because melanin radicals have a large linewidth, the low spatial
resolution of EPR imaging results in blurred images and a lack of anatomical
information. Dynamic nuclear polarization (DNP)-MRI is a
noninvasive imaging method to obtain the spatio-temporal information of free
radicals with MRI anatomical resolution. Proton signals in tissues, including
free radicals, can be dramatically enhanced by EPR irradiation at the resonance
frequency of the free radical prior to applying the MRI pulse sequence.
However, the DNP effects of free radicals in the pigment of living organisms is
unclear. Therefore, if endogenous free radicals in melanin pigment could be
utilized as a bio-probe for DNP-MRI, this will be an advantage for the specific
enhancement of melanoma tissues and might allow the separate noninvasive visualization
of melanoma tissues without the need for probe administration. Here, we report
that biological melanin pigment induced a in
vivo DNP effect by interacting with water molecules. In addition, we
demonstrated in vivo melanoma imaging
based on the DNP effects of endogenous free radicals in the melanin pigment of
living mice.Methods
To demonstrate the
capabilities of melanin radical imaging with DNP-MRI, a seven-tube phantom (200
mL, 5.4-mm diameter and 9-mm length) was prepared,
where each tube was filled with various concentrations of eumelanin in 0.2%
agarose gel water solution. The phantoms were measured by DNP-MRI with or
without EPR irradiation at 456 MHz using a surface coil. B16F0 cells (1.0 × 106/mouse)
were subcutaneously administered to the right leg of C57BL/6 mice. B16F0
bearing mice were used for the ex vivo
and DNP-MRI and EPR experiments. In vivo free radical imaging was performed with a low field (15mT)
DNP-MRI system (Keller) obtained from Japan Redox Inc. (Fukuoka Japan). In the
in vivo experiments, mice were anesthetized with 2% isoflurane at 3, 7, 10,
and 14 days after the subcutaneous injection of B16F0 cells. During the
procedure, the body temperature of the mice was kept at 37 ± 1°C with a heating
pad. The scanning conditions for the DNP-MRI experiment were as follows: power
of EPR irradiation, 7 W; flip angle, 90°; repetition time (TR) × echo time (TE) × EPR irradiation time
(TEPR), 500 × 25 ×
250 ms; number of acquisitions, 10; slice thickness, 64 mm including the whole
thickness of the mouse; phase-encoding steps, 32; field of view (FOV), 40 × 40
mm; and matrix size, 64 × 64 after reconstruction.Results and Discussion
The concentration of free radicals in melanin pigment increased
linearly as a function of the eumelanin concentration. Figure 1 shows the
DNP-MRI image of the gel phantom with various concentrations of eumelanin (2–20
mg/mL). Although melanin radicals have a large line width (4.4 ± 0.1 G) and low radical concentration in the pigment (6–58
mM), the DNP-MR image with EPR irradiation (EPR ON) showed an
enhanced MRI signal and difference images showed a dose-dependent enhancement
with eumelanin concentration. These experiments suggested that melanin free
radicals in the pigment might interact with water molecules to induce the DNP
effect.
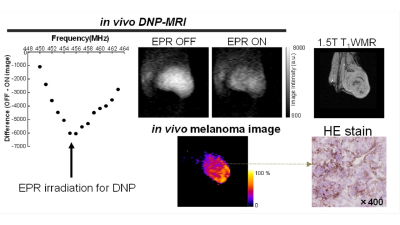
We performed the DNP-MR imaging of B16F0 bearing mice
with and without EPR irradiation at 456 MHz (Figure 1). The DNP-MRI image of the coronal
and sagittal planes and a difference map calculated using the EPR ON and OFF
images. Regular MR images (EPR OFF) demonstrated the outline of mice and the
negative enhancement in EPR ON images was clearly observed in the tumor region
of both planes(Figure 1). Difference images identified the melanoma region separately and
the melanoma regions obtained by DNP-MRI corresponded to that of 1.5T MRI
images, although the borderline tumor was not clear in the T1W MR
images. The DNP-MR image of the sagittal plane showed the depth of tumor invasion
in the leg of mice. After the imaging study, tumor tissues were extracted and frozen
sections were generated for HE staining to confirm melanin pigments by microscopy.
Dark pigments in the melanoma tissue were distributed throughout the whole
melanoma region. These experiments successfully demonstrated that
melanin radicals in the melanoma showed a DNP effect under in vivo conditions and that our system directly visualized the
melanoma including melanin free radicals with a high spatial resolution without
the need for contrast agents.Conclusion
We demonstrated in vivo melanoma imaging using
endogenous free radicals in melanin pigment using DNP-MRI. Our DNP-MRI technique
might be applied to other free radical molecules as a bio-probe for pigments or
to crystals for non-probe bio or medical imaging, if the tissues induce a DNP
effect by interactions between free radicals and water molecules.Acknowledgements
This work was supported by the Medical
Research and Development Programs Focused on Technology Transfer, Development
of Advanced Measurement and Analysis Systems (SENTAN) from the Japan Agency for
Medical Research and Development, AMED Grant Number 162128; Health Labour
Sciences Research Grant (Research on Publicly Essential Drugs and Medical
Devices) from the Ministry of Health, Labour and Welfare of Japan; and Special
Coordination Funds for Promoting Science and Technology (SCF funding program
“Innovation Center for Medical Redox Navigation”). This work was also supported
by JSPS KAKENHI (Grant Number 16H05079 and 16H05113). We thank Edanz Group
(www.edanzediting.com/ac) for editing a draft of this manuscript.References
Hyodo F, Naganuma T, Eto H, Murata M, Utsumi H, Matsuo M. In vivo melanoma imaging based on dynamic nuclear polarization enhancement in melanin pigment of living mice using in vivo dynamic nuclear polarization magnetic resonance imaging. Free Radic Biol Med. 2019 Apr;134:99-105