4754
Hypoxia alters normal fibroblast metabolism towards a cancer associated fibroblast phenotype1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Fibroblasts play a pivotal role in cancer progression. In prostate cancer, fibroblasts have been shown to induce growth, confer castration-resistance, and increase metastatic potential. To further understand how fibroblasts respond to hypoxic tumor microenvironments that are frequently observed in prostate cancer, here we have characterized the effects of hypoxia on normal prostate fibroblast metabolites as detected by high resolution 1H magnetic resonance spectroscopy. We found that hypoxia induced metabolic changes in normal prostate fibroblasts that mimicked the metabolites detected in prostate cancer associated fibroblasts (CAFs), highlighting the potential role of hypoxia in the transition of normal fibroblasts to CAFs.
Introduction
Fibroblasts are versatile cells that produce several extracellular matrix (ECM) proteins such as collagen 1 and extradomain A containing fibronectin, as well as degradative enzymes such as matrix metalloproteinases1. Fibroblasts play an important role in wound healing, and in different pathological conditions such as the extensive fibrosis observed in chronic conditions, and in cancer progression. Cancer associated fibroblasts (CAFs) are directly involved in tumor progression and dissemination2-4. We recently identified increased CAFs in more metastatic prostate cancers5. To understand the influence of hypoxia that is frequently observed in prostate cancer in modifying fibroblast metabolism, here we characterized human prostate fibroblast metabolites as detected by 1H MRS under normoxic and hypoxic conditions and compared these to metabolites detected in CAFs obtained from human prostate cancer.Methods
Experiments were performed using human prostate fibroblasts (WPMY-1, ATCC, Manassas, VA) and human prostate cancer associated fibroblasts (PCAFs, Asterand Bioscience, Detroit, MI). WPMY-1 were derived from stromal cells from the peripheral zone of the histologically normal adult prostate6. PCAFs were obtained from an adenocarcinoma of the prostate gland. In order to induce hypoxia, WPMY-1 cells were incubated for 48h under hypoxic conditions (0% O2) using an induction chamber where all the oxygen was displace by 100% N2 at the start of incubation. For high resolution 1H MRS, cell extracts were obtained using a dual-phase extraction method as previously described7. Water-soluble samples were dissolved in 0.6 mL of buffered D2O (Sigma). High-resolution 1H MR spectra were recorded on a Bruker Biospin Avance-III 750 MHz NMR (Bruker Biospin) spectrometer operating at a proton frequency of 750.21 MHz using a 5-mm broad band inverse (BBI) probe head equipped with z-gradient accessories. 1H MR spectra were manually phased and automated baseline corrected using TOPSPIN 3.2 software. Integrals of the metabolites of interest were determined and normalized to the TSP reference and the number of cells. Metabolites were estimated from at least four experimental samples. Statistical significance was evaluated using the Student t test.Results and discussion
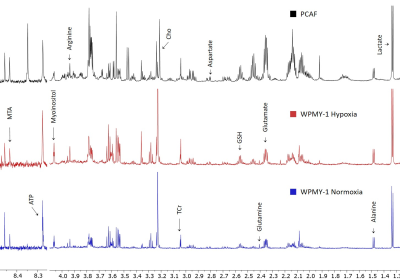
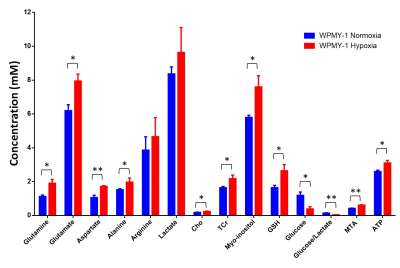
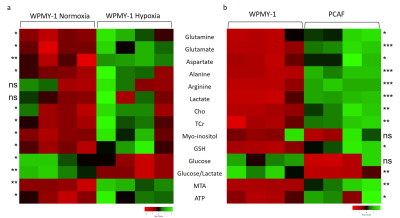
We have previously observed that hypoxia triggered significantly faster degradation of the ECM by fibroblasts8. Here, we found that a 48h incubation of normal prostate fibroblasts under hypoxia significantly altered several metabolites making the spectra more similar to spectra obtained from PCAFs under normoxic conditions, as shown in the representative spectra in Figure 1. Significance metabolic differences between normoxia and hypoxia, summarized in Figure 2, included glutamine, glutamate, aspartate, alanine, choline (Cho), total creatine (TCr), myo-inositol, reduced glutathione (GSH), glucose, S-methyl-5'-thioadenosine (MTA), and ATP. The metabolic similarities between normal WPMY-1 under hypoxia and PCAFs can be identified by comparing the heat maps in Figures 3a and 3b. These data suggest that hypoxia plays an important role in the metabolic transformation of fibroblasts to a malignant metabolic phenotype. Future studies with normal fibroblasts and CAFs obtained from different cancers should further validate the role of hypoxia in the metabolic transformation identified in this study.Acknowledgements
This work was supported by NIH R35CA209960 and R01CA82337. JPT was funded by Fundación Alonso Martín Escudero and MSCA.References
1. Zent J and Guo LW. Signaling Mechanisms of Myofibroblastic Activation: Outside-in and Inside-Out. Cell Physiol Biochem. 2018; 49(3):848-868.
2. Glentis A, Oertle P, Mariani P, et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat Commun. 2017; 8(1):924.
3. Labernadie A, Kato T, Brugues A, et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017; 19(3):224-237.
4. Valencia T, Kim JY, Abu-Baker S, et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014; 26(1):121-135.
5. Penet MF, Kakkad S, Pathak AP, et al. Structure and Function of a Prostate Cancer Dissemination-Permissive Extracellular Matrix. Clin Cancer Res. 2017; 23(9):2245-2254.
6. Webber MM, Trakul N, Thraves PS, et al. A human prostatic stromal myofibroblast cell line WPMY-1: a model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis. 1999; 20(7):1185-92.
7. Penet MF, Shah T, Bharti S, et al. Metabolic imaging of pancreatic ductal adenocarcinoma detects altered choline metabolism. Clin Cancer Res. 2015; 21(2):386-95.
8. Pacheco-Torres J, Shah T,. et al. Investigating the effects of hypoxia on fibroblast invasion and metabolism. ISMRM 2019, abstract # 2300
Figures