4752
Imaging and pain response in extra vs intra-pelvic recurrent gynaecological tumours treated with MR guided HIFU: a pilot study1Imaging, Institute of Cancer Research, Sutton, Surrey, United Kingdom, 2Clinical Oncology, The Royal Marsden NHS Foundation Trust, London, United Kingdom, 3MRI Unit, The Royal Marsden NHS Foundation Trust, Surrey, United Kingdom, 4Therapy Ultrasound, Institute of Cancer Research, Sutton, Surrey, United Kingdom
Synopsis
MR guided High Intensity Focused Ultrasound in 10 patients with symptomatic recurrent gynecological tumors treated for pain palliation resulted in higher temperatures in shallower extra-pelvic tumors compared to intra-pelvic ones (62.9±11.7oC vs. 49.4±2.8oC over median 16 sec sonication) despite lower energy delivery in them (39.1±23.6 kJ vs. 72.1±24.3 kJ). In 3 of 5 extra-pelvic tumors, an increase in the non-perfused tumor volume immediately after treatment was seen, indicating edema. At Day 30, 5 patients met the criteria of pain response, 3 with extra-pelvic tumors and 2 with intra-pelvic tumors.
Background
MR guided HIFU (MRgHIFU) is used in the clinic for ablating uterine fibroids, but its role in treating malignant gynecological tumours is not established. The location and depth of tumors relative to fat and muscle can preclude effective delivery of thermal energy (1). We have previously established the feasibility of targeting these tumors in a planning study (2).Aim
To conduct a pilot feasibility study treating recurrent gynecological tumours palliatively with MRgHIFU, to establish the changes in tumor volume and contrast enhancement patterns of extra- vs intra-pelvic tumors and to relate them to thermal dose delivered and pain outcomes at 30 days.Methods
In a prospective trial, 10 patients (11 treatments) with painful recurrent gynecological tumours underwent MRgHIFU. Treatment was delivered as a series of exposures (sonications) using a Philips Sonalleve V2 system integrated with a 3T Achieva. T1- and T2-W imaging in the axial plane and pre- and post-contrast 3D-mDIXON sequences before and after gadolinium enhancement were obtained up to 3 weeks pre-treatment, immediately post treatment and at 7- and 30-days post-treatment. Total thermal energy delivered varied with patient positioning, depth of lesion and its location and proximity to adjacent organs. The whole tumor and the non-perfused tumor volume were delineated by regions-of-interest on every slice at each time-point; enhancing lesion volume was obtained by subtraction. Ratios of enhancing to whole lesion volumes were calculated. Pain scores from questionnaires, and analgesic use were recorded. A significant improvement in pain was identified as a greater than 2-point reduction in pain score or a 25% reduction in analgesic use.Results
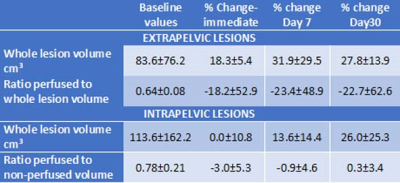
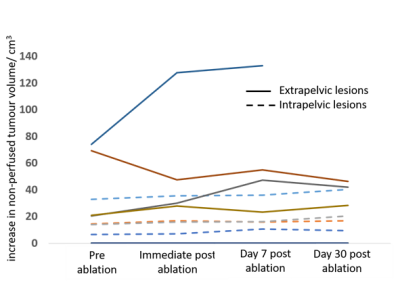
Tumor volumes ranged from 1.7-444.0 cm3 at baseline. Locations were intra-pelvic in 5 cases (6 patient treatments, 1 post-exenteration) and extra-pelvic in 5 cases (3 inguinal, 1 ischiorectal fossa, 1 popliteal fossa). 1 extra-pelvic tumor was ablated entirely (no longer visible following contrast administration); the other 4 all showed an immediate increase in total tumor volume (18.3±5.4%). Intra-pelvic tumors did not show significant increase in volume immediately post-treatment (0±10.8%). At Day 7 and 30 both extra-pelvic and intra-pelvic tumors demonstrated an increase in volume (Table 1). Total energy delivered was lower in the extra-pelvic tumors (39.1±23.6 kJ), which were at shallower depth than in the deeply sited intra-pelvic ones (72.1±24.3 kJ). Also, the average of the maximum temperature recorded over each sonication (16 secs - 40 secs, median 16 secs) was higher for extra-pelvic tumors (62.9±11.7oC vs. 49.4±2.8oC).Ratios of enhancing to whole tumor volumes at baseline ranged from 0.47-1.0. Immediately post-treatment there was a decrease in this ratio for extra-pelvic tumors, but no change in the intra-pelvic ones. However, variability in the extra-pelvic tumors was high (Table 1). Data for the change in non-perfused volume for individual patients is illustrated in Figure 1.
At Day 30, 5 patients met the criteria of response, 3 with extra-pelvic tumors and 2 with intra-pelvic tumors. Non-response was recorded in 2 patients with extra-pelvic tumors (1 that suffered a skin burn) and in 3 patients (4 treatments, 1 patient treated twice) with intra-pelvic tumors.
Discussion and Conclusions
Due to restrictions in the available device settings, it was difficult to achieve ablative thermal doses in deep pelvic tumors using the Sonalleve hardware. Where thermally ablative temperatures (>55oC) were achieved in the extra-pelvic tumors, there was an immediate increase in tumor volume, mainly in the non-perfused compartment, indicative of edema. Despite lower temperatures with the increased energy delivered in intra-pelvic compared to extra-pelvic tumors, there was a symptomatic improvement in pain in 2 patients with intra-pelvic tumors, which may be attributable to neural damage at the lower temperatures (3) or to a placebo effect. Nine of 10 tumors increased in volume with time indicating tumour progression, although alterations in the pace of progression cannot be estimated in this pilot study in the absence of a randomized control group. Nevertheless, an improvement in symptoms was achieved in 45% of treatments in this palliative care setting.Acknowledgements
NHS funding from the NIHR Research for Patient Benefit programme (PB-PG-0815-20001), and to the NIHR Biomedical Research Centre, the Clinical Research Facility in Imaging and the Cancer Research Network.
CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC & Department of Health C1060/A10334, C1060/A16464.
We would also like to acknowledge the support of Philips Healthcare, Profound Medical and the Focused Ultrasound Foundation.
References
1) Giles SL et al Proc ISMRM 2019, Montreal, #3813
2) Giles SL et al, Br J Radiol. 2019; 92(1098):20181037.
3) Lee YF et al, Br J Anaesth. 2015;114: 840-6.