4750
Improving tumor-tissue contrast by increasing spatial resolution1Radiology, University of Cincinnati, Cincinnati, OH, United States, 2Brain Tumor Center, University of Cincinnati, Cincinnati, OH, United States, 3Pediatrics, University of Cincinnati, Cincinnati, OH, United States, 4The Perinatal Institute and Section of Neonatology, Perinatal and Pulmonary Biology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 5Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 6Radiation Oncology, University of Cincinnati, Cincinnati, OH, United States
Synopsis
There is broad agreement that increased tumor-tissue contrast significantly improves tumor visibility. It is not yet clear whether increasing spatial resolution will improve tumor-tissue contrast. In this study, we found that the increasing spatial resolution can significantly increase tumor-tissue contrast when tumor size is comparable to the spatial resolution, but provides an invariant contrast when tumor size is much larger than the spatial resolution.
Introduction
There is broad agreement that increased tumor-tissue contrast significantly improves tumor visibility. Most recently, ultra-high field magnetic resonance imaging (MRI) has been used to improve lesion-tissue contrast.1-3 With the assumption that voxel signal comes from a homogenous portion of tissue within the voxel, tumor-tissue contrast is invariant as a function of spatial resolution/voxel size.4 What is not yet clear is the effect of spatial resolution/voxel size on the tumor-tissue contrast without the above homogeneity assumption. In this study, we set to discuss such effect both theoretically and experimentally, and then explore potential pathway to improve tumor-tissue contrast, thereby facilitating diagnostic confidence and accuracy in contrast-enhanced MR imaging (CE-MRI).Theory
For CE-MRI brain tumor imaging, if a tumor voxel i with the voxel size of includes enhanced tumor cells E and non-enhanced cells N, the combined signal from the tumor voxel is the summed fractional signals of SE(i) and SN(i). The contrast between tumor voxel i and normal tissue voxel j is given by: $$contrast_{\triangle v}=[a_{i}S_{E(i)}+(1-a_{i})S_{N(i)}]-S_{N(j)}$$ (1)where αi is defined as fraction of enhanced tumor cells. Let’s assume the signals from both non-enhanced cells and the enhanced tumor cells are homogeneous, respectively, that is SN(i) = SN(j) = SN and SE(i) = SE, then equation (1) can be simplified to:
$$contrast_{\triangle v}=a_{i}(S_{E}-S_{N}) $$ (2)
Therefore, tumor-tissue contrast in a multi-voxel tumor region can be given by:
$$contrast_{\triangle v}=(S_{E}-S_{N})\frac{1}{n}\sum_i^na_{i}$$ (3)
where n is total number of voxels in the tumor region. We assume that the αi is a constant α inside the tumor region, while at the tumor-tissue boundary, partial volume effect will lead to a variation of fraction αk with reduced value (i.e. αk < α ). then
$$contrast_{\triangle v}=(S_{E}-S_{N}) [\frac{N_{in}}{n}\alpha+\frac{1}{n}\sum_a^m\alpha_{i}]$$ (4)
where Nin and m are number of voxels inside the tumor region and at the tumor-tissue boundary, respectively. When the voxel size is reduced from ΔV to βΔV (β<1), total number of voxels in the tumor region will become from n to n'/β , and then signal intensities become βSE for enhanced tumor and βSN for non-enhanced tissue. The contrast with reduced voxel size can be given by: $$contrast_{β\triangle v}=(S_{E}-S_{N})β[\frac{N'_{in}}{n'}\alpha+\frac{1}{n'}\sum_a^m'\alpha_{i}]$$ (5)
where N'in and m' are the voxel number inside the tumor region and at the tumor-tissue boundary, respectively. The contrast change caused by the increasing spatial resolution is $$contrast_{β\triangle v} - contrast_{\triangle v} ≥ (S_{E}-S_{N}) [(\frac{N'_{in}}{n'}\beta-\frac{N_{in}}{n})\alpha+(\frac{\beta}{n'}\sum_k^m'a_{k}-\frac{1}{n}\sum_i^m\alpha'_{i})]$$
(6)
When the tumor region is comparable to the voxel size, almost all voxels occur at the tumor-tissue boundary. The contrast variation in Equation (6) is dominated by $$(\frac{\beta}{n'}\sum_k^m'a_{k}-\frac{1}{n}\sum_i^m\alpha'_{i}) $$. The reduced voxel size may result in increasing the summed fraction of enhanced tumor. Thus, the reduced voxel size or increasing spatial resolution will lead to the increased tumor-tissue contrast for the small tumor.
Method
In vivo experiment: Eight patients with brain tumors (4 pituitary tumors and 4 brain metastases) were scanned with a randomized spatial resolution acquisition order using a conventional three dimensional (3D) CE-MRI sequence 5 with isotropic resolutions of 1.2, 1.0, and 0.9 mm, after contrast administration of 0.1 mmol/kg Gadavist (Bayer HealthCare) on a General Electric Signa Architect 3.0 T scanner.Quantitative evaluation:$$ |\frac{S_{A}-S_{B}}{S_{A}+S_{B}}|$$ We experimentally estimated tumor-tissue image contrast by calculating , where A was a randomly selected enhanced tumor region (if there were multiple lesions) and B was a normal tissue region near the selected region A.
Statistical Analysis: Linear mixed models were used to compare the measured contrast obtained using different resolutions for each patient. Box plots were performed to illustrate the distribution of tumor-tissue contrast for each isotropic resolution. All analyses were performed using SAS version 9.4, with p-values less than 0.05 were considered statistically significant.
Results
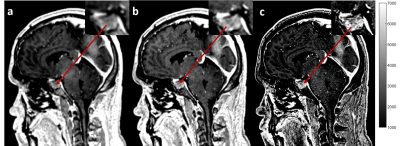
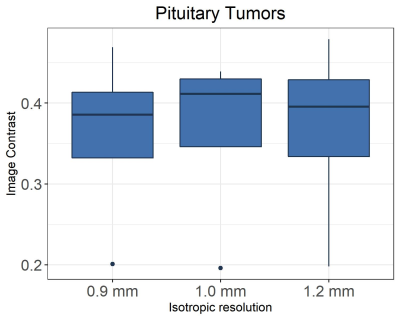
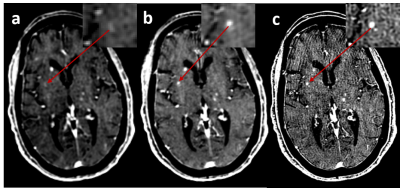
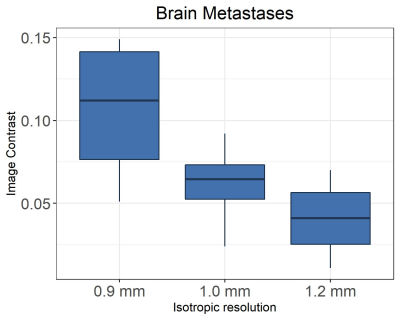
Figure 1 shows in vivo brain images of a patient with pituitary tumor acquired with the different spatial resolutions. Visually, higher spatial resolution provides better definition of the pituitary tumor architecture and near-invariant tumor-tissue contrast, as shown in Figure 2.Figure 3 shows the enhanced brain metastasis (red arrows) is more visually evident with higher resolution. Quantitative analysis in Figure 4 indicates that tumor- tissue contrasts are 0.07, 0.092, and 0.149 for the isotropic resolutions of 1.2, 1.0 and 0.9 mm, respectively. Tumor-tissue contrast statistically significantly increases with increasing spatial resolution for brain metastases.
Discussion and conclusions
In our study, tumor size was 8-18 mm for pituitary tumors and 2-5 mm for brain metastases. Increasing spatial resolution reduces the partial volume effect which leads to an increased summed fraction of enhanced tumors at the tumor-tissue boundary (Equation 6). As a result, the increasing spatial resolution enhances the tumor-tissue contrast for the tumor with smaller sizes. However, for larger tumors, the number of voxels inside the tumor region is much more than the number of voxels at the tumor-tissue boundary. Thus, the partial volume effect can be ignored and the increasing spatial resolution lead to an invariant tumor-tissue contrast. Our in-vivo experimental results are in a good agreement with our theoretical analysis. In CE-MRI, we demonstrated that the increasing spatial resolution results in an invariant tumor-tissue contrast when tumor size is much larger than the spatial resolution, and a significant increased tumor-tissue contrast when tumor size is comparable to the spatial resolution.Acknowledgements
This study was supported by CincyTech Technology Commercialization Accelerator Award and Pilot Pilot Grant funding from University of Cincinnati Brain Tumor Center.References
1. Obusez, E.C., et al. 7T MR of intracranial pathology: Preliminary observations and comparisons to 3T and 1.5T. NeuroImage 168, 459-476 (2018).
2. Karamat, M.I., Darvish-Molla, S. & Santos-Diaz, A. Opportunities and Challenges of 7 Tesla Magnetic Resonance Imaging: A Review. 44, 73-89 (2016).
3. Maranzano, J., et al. Comparison of Multiple Sclerosis Cortical Lesion Types Detected by Multicontrast 3T and 7T MRI. American Journal of Neuroradiology 40, 1162-1169 (2019).
4. Brown, R.W., Cheng, Y.-C.N., Haacke, E.M., Thompson, M.R. & Venkatesan, R. Magnetic resonance imaging: physical principles and sequence design, (John Wiley & Sons, 2014).
5. Ellingson, B.M., et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro-Oncology 17, 1188-1198 (2015).
Figures