4744
Magnetic Resonance Elastography Predicts Early Resistance to Chemotherapy in Cancer1School of Cancer and Pharmaceutical Sciences, King's College London, London, United Kingdom, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 3Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom, 4Laboratory of Vascular Translational Science, UMR1148,, INSERM-University Paris Diderot, Paris, France, 5UCL Cancer Institute, University College London, London, United Kingdom
Synopsis
There is an unmet need in cancer treatment for a non-invasive technique capable of identifying drug resistance very early in the therapeutic cycle. Our team has found that tumoral tissue changes its physical aspect as a response to therapeutic stress and that MRE can detect such changes by probing tissue biomechanics. Using a chemotherapy resistant pre-clinical model, we showed that tumors solidify as a resistance mechanism. Changes in the biomechanics were then correlated with changes Collagen and Hyaluronic acid depositions, both of which can affect the immune response.
Introduction
Squamous cell carcinomas of the head and neck (HNSCC) includes cancers that occur in different sites in the head and neck area including lips, oral cavity, nasal cavity, salivary glands, paranasal sinuses, thyroid pharynx and larynx. It represents the fifth most common cancer with around 15 thousand new cases a year majority. Mortality is estimated at 4 thousand a year and as such is one of the highest in Europe 1. Despite having relatively low rate of distant metastasis of 12%, 5-year survival is low ranging between 28 and 57 % with minimum improvement over the last 20 years. Local recurrence following first line treatment occurs in over 30% of the patients despite initial response. Chemotherapy, mainly cisplatin is given to patients with recurrence to which only a small proportion of the patients will respond. The ability to stratify patients early on in the course of their therapy to determine responders from those who could benefit from changes in treatment regimen such as an inclusion of an immunotherapy is severely lacking 2.Material and Methods
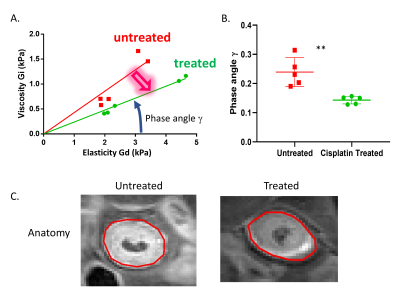
Balb/c SCID mice were xenografted by subcutaneous injections with a human patient derived head and neck cancer cell line resistant to chemotherapy. Resistance was confirmed by PI Annexin V flow cytometry, and tissue analysis of the stroma by immunofluorescence. Imaging was done with a 9.4T Bruker Biospec, a spin echo sequence was used for anatomical images and a modified spin echo sequence for elastography acquisitions with a vibration frequency of 500 Hz. Calculations for the complex shear modulus |G*|, elasticity Gd, viscosity Gl and the phase angle were calculated as previously described 3,4Results
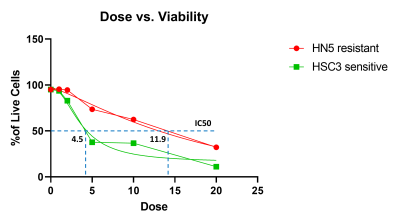
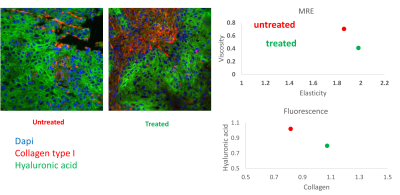
Viability analysis after 4 days of culture with increasing concentrations of Cisplatin revealed that the resistant HN5 cell line has an IC50 of more than double of a sensitive. In-vivo data not shown here shows complete shrinkage of the sensitive cell line xenografts. Anatomical MRI scans did not show a significant change in the size of the chemo-therapy resistant tumor with Cisplatin treatment. However, quantification of the biomechanical properties of the tumoral tissue revealed an increase in elasticity with a concomitant decrease in viscosity in the treated tumours compared to the untreated ones. Furthermore, analysis of the viscoelastic phase angle revealed both a significant decrease and a normalization of the values. Whereas the phase angle ranged between ranged for 0.19 and 0.31 for untreated mice, the values of the treated ones ranged between 0.13 and 0.15. Immunoflourescence staining for type 1 Collagen (Col1) and Hyaluronic acid (HA) showed that the stroma is mainly formed of mutually exclusive areas either rich for one or the other. Quantification showed an increase in Collagen rich area coupled with a decrease of HA rich as a function of treatment.Discussion
Phase angle reflects the solid/liquid aspects of the tissue. Previous work has shown that an increase in phase angle correlates with therapeutic success5. In this model we used a therapy resistant model to show that tissue biomechanical changes can be indicative of resistance to therapeutic stress. The two key aspect to consider are first, the decrease in the phase angle showing that the tissue is solidifying as a potential mechanism of resistance. The second is the decrease in the variance of the solid/liquid aspect of the tissue which shows that the biological response to chemotherapy induced stress is not only uniform but independent of the un-treated resting state of the tissue. Interestingly these changes are observed early in the treatment cycle and as such can serve as a stratification tool for responders in the clinical settings. Analysis of the stroma was performed to link the changes in elasticity and viscosity to a molecular component. Collagens particularly type I collagens are the most abundant proteins in the ECM, their main function is to provide tissue rigidity and as such can directly impact the stiffness readout of MRE 6. Hyaluronic acid on the other hand is a very long negatively charged polysaccharide. Its main function is to act as a cushion and dissipate forces impact the tissue by accumulating water molecules 7. Both of the components have the ability to elicit an immune response under pathological conditions and are reciprocally regulated by the same immune response 8.Conclusion and Future perspectives:
Molecular changes in cancer driven by therapeutic stress can change the physical aspects of the tissue. MRE is a viable non-invasive technique that allows to quantify such changes and eventually correlate them with therapeutic outcome. As changes in the stroma are driven by the cellular components, future work will investigate changes in fibroblasts and immune cells to fully understand the underlying mechanisms. As immunodeficient mice have their limits, we intend to use syngeneic HNSCC models. Finally, immunotherapy has recently been approved for use in HNSCC. We hope that the use of MRE early in the course of the chemotherapy could identify non-responders who would benefit from a combination with such therapeutics.Acknowledgements
No acknowledgement found.References
1. Lafuma, A. et al. Economic burden of chemotherapy-treated recurrent and/or metastatic squamous cell carcinoma of the head and neck in France: real-world data from the permanent sample of national health insurance beneficiaries. J Med Econ 22, 698-705, doi:10.1080/13696998.2019.1594837 (2019).
2. Manikantan, K. et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treatment Reviews 35, 744-753, doi:10.1016/j.ctrv.2009.08.007 (2009).
3. Sinkus, R. et al. MR elastography of breast lesions: Understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magnetic Resonance in Medicine 58, 1135-1144, doi:10.1002/mrm.21404 (2007).
4. Pagé, G. et al. Assessing Tumor Mechanics by MR Elastography at Different Strain Levels. J Magn Reson Imaging, doi:10.1002/jmri.26787 (2019).
5. Li, J. et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer 110, 1727-1732, doi:10.1038/bjc.2014.76 (2014).
6. Li, J. et al. Investigating the Contribution of Collagen to the Tumor Biomechanical Phenotype with Noninvasive Magnetic Resonance Elastography. Cancer Res, doi:10.1158/0008-5472.CAN-19-1595 (2019).
7. Voutouri, C. & Stylianopoulos, T. Accumulation of mechanical forces in tumors is related to hyaluronan content and tissue stiffness. Plos One 13, 14, doi:10.1371/journal.pone.0193801 (2018).
8. Lee-Sayer, S. S. M. et al. The where, when, how, and why of hyaluronan binding by immune cells. Frontiers in Immunology 6, 12, doi:10.3389/fimmu.2015.00150 (2015).
Figures