4743
Biomechanical Response of Orthotopic Pancreatic Tumours to the Vascular Disrupting Agent ZD6126 Assessed by Magnetic Resonance Elastography
Jin Li1, Emma L. Reeves1, Jessica K.R. Boult1, Craig Cummings1, Jeffrey C. Bamber1, Ralph Sinkus2, Yann Jamin1, and Simon P. Robinson1
1Division of Radiotherapy & Imaging, The Institute of Cancer Research, London, United Kingdom, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, King’s Health Partners, St. Thomas’ Hospital, London, United Kingdom
1Division of Radiotherapy & Imaging, The Institute of Cancer Research, London, United Kingdom, 2Division of Imaging Sciences and Biomedical Engineering, King's College London, King’s Health Partners, St. Thomas’ Hospital, London, United Kingdom
Synopsis
MRE-derived shear stiffness |G*| of orthotopic pancreatic tumours was sensitive to a heterogeneous treatment response induced by the vascular disrupting agent ZD6126.
Introduction / Purpose
Stromal-dense pancreatic ductal adenocarcinoma (PDAC) is characterized by extensive deposition of extracellular matrix (ECM) components, loss of tensional homeostasis and hypovascularity, impairing efficient drug delivery.1-3 Collagen, the principal component of the fibrillary protein network within the ECM, is the major contributor to ECM stiffening, is strongly implicated in tumour evolution and progression, and is associated with poor patient prognosis. Patients with pancreatic cancer continue to have poor 5-year survival rates of ~3%. A major contributory factor to this dismal prognosis is the lack of early diagnosis using current imaging techniques/biomarkers, as well as limited treatment options.Magnetic resonance elastography (MRE) is an emerging imaging technique being used to directly visualise and quantify the viscoelastic properties of tumours in vivo. We recently demonstrated the use of MRE, coupled with computational pathology, to interrogate the contribution of collagen to the tumour biomechanical phenotype in a range of pre-clinical tumour models in vivo4. The study highlighted that the markedly high stiffness quantified by MRE in orthotopically-propagated PDAC tumours was associated with high collagen deposition, providing a strong rationale for using MRE to assess the response of PDAC to ECM-targeted drugs. As a prelude to this, MRE was used to quantify the elasticity and viscosity of orthotopic PDAC xenografts prior to and following treatment with the vascular disrupting agent ZD6126, previously shown to induce extensive tumour necrosis in a range of pre-clinical tumour models.5,6
Methods
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Anaesthetised CD1 nu/nu mice bearing orthotopic PDAC tumours derived from PANC-1 cells were imaged prior to and 24 hours after treatment with 200 mg/kg ZD6126, using a 3 cm birdcage coil on a 7T Bruker MicroImaging horizontal MRI system (Bruker Instruments, Ettlingen, Germany). MRE data were acquired in the axial plane and a purpose built platform as previously described.6 Maps of the absolute value of complex shear modulus |G*| (G* = Gd + iGl, Gd: elasticity, Gl: viscosity, all in kPa) and the loss tangent Gl/Gd were reconstructed with an isotropic pixel size of 300 µm, and mean |G*| were determined from a region of interest covering the tumour. Paraffin-embedded tissue sections were cut and stained with H&E (necrosis), picrosirius red (collagen I&III) and endomucin (microvasculature). Images of picrosirius red stained sections were also acquired with polarized light microscopy to investigate collagen fibre architecture.Results
Orthotopic PDAC xenografts revealed a heterogeneous regional distribution of |G*|, characterised by foci of elevated values. Treatment with ZD6126 over 24 hours resulted in regional reduction in |G*| in all three tumours (Fig. 1 & 2A). ZD6126-induced regional reductions in |G*| were associated with expansive areas of low endomucin staining and necrosis, surrounded by a thin rim of viable cells (Fig. 3). In the largest tumour from mouse #1, the change in viscoelastic properties was spatially more heterogeneous, with regions showing the smallest reduction in |G*| corresponding to areas of viable tumour with markedly higher collagen deposition (Fig. 3). Detection of endomucin-stained microvasculature post-treatment was restricted to the tumour periphery. Elevated signal in the loss tangent Gl/Gd maps was spatially associated with thick collagen fibre deposition revealed by polarized light microscopy, while there were regions of high |G*| value associated with relatively low collagen fibre deposition and dense viable cellularity (Fig. 4).Discussion
Treatment with the vascular disrupting agent ZD6126 induced a clear reduction in the shear modulus |G*| of orthotopic PANC-1 xenografts associated with pathologically-confirmed tumour necrosis. This response is consistent with response to vascular disrupting agents in other pre-clinical tumour models following treatments6,7, and highlights that MRE can provide sensitive biomarkers of treatment response in this model of PDAC.Tumour viscoelastic properties are determined by cellularity, vascularity, and ECM components.6,8 Generally, responsive regions of PANC-1 tumours exhibited relatively high |G*| values pre-treatment. It is thus reasonable to assume that these regions represented viable tumour tissue with relatively patent vasculature and collagen network before treatment, as previously observed.4 In comparison, regions that showed no MRE response to ZD6126 were mostly avascular, with viable tissue supported by a dense collagen network. The absence of any detectable microvasculature within these non-responding regions reiterates the negative impact of the stromal dense microenvironment of PDAC on efficient drug delivery.
Interestingly, the case study from mouse #1 illustrated that the less studied MRE-derived parameter loss tangent Gl/Gd, related to energy dissipation, appeared to reflect the distribution of collagen fibres, while |G*| value was affected by both cell viability and collagen deposition.
PDAC is considered hypovascular, so the marked MRE-response and induction of necrosis following treatment was somewhat surprising, as ZD6126 targets established tumour blood vessels.5 A similar pathological response to ZD6126 was reported in orthotopic L3.6pl pancreatic xenografts.9 Collectively these data suggest that therapeutic targeting of the vascular compartment in PDAC may be an effective treatment strategy.
Conclusions
Quantitation of the shear modulus |G*| with MRE provides a sensitive biomarker of the extent and heterogeneous response of stromal rich orthotopic PDAC xenografts to the vascular disrupting agent ZD6126 in vivo. The study provides a rationale for performing MRE-embedded pre-clinical trials of novel therapeutics that target the ECM being developed for the treatment of PDAC, a cancer of unmet need.Acknowledgements
We acknowledge funding from the Cancer Research UK Imaging Centre at the ICR, and funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039, and Cancer Research UK Centre at the ICR, the Cancer Research UK grant C16412/A27725, in association with a Children with Cancer UK Research Fellowship.References
- Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut 2011; 60: 861-868.
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2007; 6: 1186-1197.
- Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 2015; 12: 319-334.
- Li J, Zormpas-Petridis K, Boult JK, et al. Investigating the Contribution of Collagen to the Tumor Biomechanical Phenotype with Non-invasive Magnetic Resonance Elastography. Cancer Research 2019.
- Blakey DC, Westwood FR, Walker M, et al. Antitumor activity of the novel vascular targeting agent ZD6126 in a panel of tumor models. Clin Cancer Res 2002; 8: 1974-1983.
- Li J, Jamin Y, Boult JK, et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer 2014; 110: 1727-1732.
- Jugé L, Doan B-T, Seguin J, et al. Colon tumor growth and antivascular treatment in mice: complementary assessment with MR elastography and diffusion-weighted MR imaging. Radiology 2012; 264: 436-444.
- Jamin Y, Boult JK, Li J, et al. Exploring the biomechanical properties of brain malignancies and their pathologic determinants in vivo with magnetic resonance elastography. Cancer Res 2015; 75: 1216-1224.
- Kleespies A, Kohl G, Friedrich M, et al. Vascular targeting in pancreatic cancer: the novel tubulin-binding agent ZD6126 reveals antitumor activity in primary and metastatic tumor models. Neoplasia 2005; 7: 957-966.
Figures
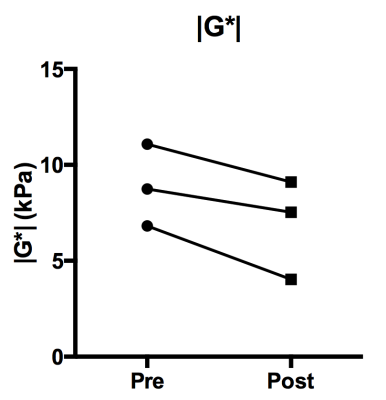
Figure 1.
Ladder plot showing the individual response of the shear modulus |G*| obtained
from orthotopically propagated PDAC xenografts to treatment with ZD6126.
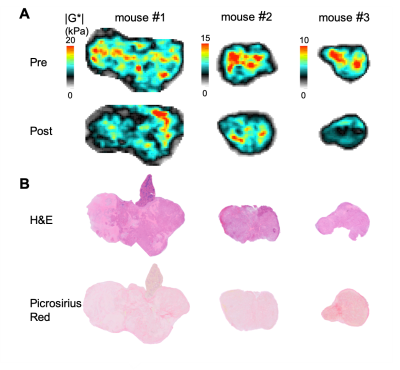
Figure 2. (A) Parametric maps of the shear modulus |G*| obtained from orthotopic
PDAC xenografts prior to and 24 hours after administration of 200 mg/kg ZD6126,
and (B) composite images of haemotoxylin
and eosin (H&E), and picrosirius red stained sections obtained from the
same tumour.
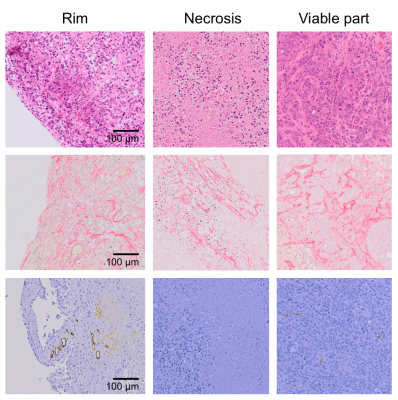
Figure 3. Microscopic images of sections stained with
H&E (top row), picrosirius red (middle row), and for endomucin (bottom row)
from the tumour rim, and from areas of necrosis and viable tumour
post-treatment, revealing regional histopathological heterogeneity.
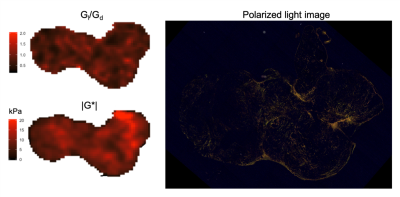
Figure 4. Parametric maps of the loss tangent
Gl/Gd and |G*| acquired from the orthotopic PDAC tumour
in mouse #1 after ZD6126 treatment, and a polarized light image from a picrosirius
red stained section from the same tumour.