4729
Imaging of Stroke in Rodents using a Clinical Scanner and Implanted Inductively Coupled Receiver Coils1NeosBiotec, Pamplona, Spain, 2Neuroscience, Fundación para la Investigación Médica Aplicada, Pamplona, Spain, 3Radiology, Clínica Universidad de Navarra, Pamplona, Spain
Synopsis
We and others have previously shown that clinical scanners can be employed to image small laboratory animals using wireless inductively coupled coils (ICC). In this work, we evaluated the use of implanted ICCs for the same purpose and demonstrated that they can provide high SNR images that allow longitudinal assessment of stroke lesions in rodents.
Introduction
Imaging techniques have become important tools in translational research. Among then, preclinical MRI using dedicated high-field scanners can provide high-quality images of laboratory animals. However, access to these systems is limited due to their cost. For some applications, a clinical scanner can offer a useful alternative for preclinical research, despite the lower field and gradient strengths. The authors1 and others2,3 have shown that high SNR images of small animals can be acquired in a 3T scanner, using especially designed receiver coils. In previous work we presented surface and volume inductively coupled coils (ICC) and demonstrated that used in combination with a sniffer coil, they can yield high quality images of the rodent brain. Further SNR gains should be achieved by employing implantable ICCs4,5, since the dimensions of the coil can be reduced, and the coil can be placed closer to the structure of interest. The goal of this study was to extend our previous work by designing and testing implantable ICCs to image rat and mouse brain in a 3T scanner. In this work, we evaluated the custom-made coils in rat and mouse models of stroke.Methods
RF Coils: The ICC consisted of a wireless, inductively coupled resonant loop4, tuned to the Larmor frequency, and used in combination with a sniffer coil that transferred the signal to the scanner receiver. To maintain the homogeneity of the B1+ transmit field, the wireless loop was fitted with a passive detuning circuit. Different circuit designs were studied: antiparallel limiter diode pair, PIN diode biased by RF rectifying circuit, Enhancement Mode Gallium Nitride field effect transistor and Schottky diode pair. The effect of the detuning tank circuit in the Q spoiling was also analyzed and tested, leading to the conclusion that this tank circuit is not particularly useful for our passive detuning application, due to the non-linear behavior of the RF switches, which is power-level dependent. Different dielectric encapsulation materials were also tested, being the final choice a dental cement which provided the required isolation with the minimum effect on SNR. The coil design also incorporated a non-magnetic trimmer to allow a final adjustment of the resonant frequency after being implanted on the animal.Animals: One adult male Sprague-Dawley rat (250-300 g) and one C57BL/6J mouse were used. Animal care and procedures were approved by the ethics committee of the University of Navarra.
Infarct induction: The animals were anesthetized and a longitudinal incision along the midline scalp was performed. Then, a dose of 150mg/kg of Rose Bengal was injected intraperitoneally. After five minutes, a cold light beam was applied for 30 minutes by direct contact to the skull.
Coil implantation: Once the infarct induction was completed, we proceeded with the coil implantation (Fig. 1a). To do that, the skull was first covered with a thin layer of dental cement. This layer served to increase the adherence of the implant. Then the coil was placed and temporary fixed with several drops of cement. Once the coil was conveniently placed the whole system (coil and electronic elements) was completely covered with additional dental cement; only the trimmer was accessible.
Scanning protocol: The animals were scanned under anesthesia one day after infarct induction. In addition, the mouse was re-scanned after one week. The rat was placed in prone position on a custom-built support, which was introduced inside the clinical wrist-coil (Fig. 1b). The mouse was placed in prone position over the manufacturer’s spine coil (Fig. 1c). Imaging was performed on a 3T-Siemens Skyra (45 mT/m gradient strength) using the body-coil for RF transmission. The resonant frequency of the ICC was adjusted prior to the imaging study. The scanning session included localizers and T2-weighted sequences (see parameters in Table 1), run in coronal orientation.
SNR measurements: An ROI was drawn in the cortex, contralateral to the infarcted side. SNR was calculated as the ratio of the mean ROI signal to the standard deviation of the background signal.
Histology: Brain fixation was achieved by transcardial perfusion with paraformaldehyde(PFA) under anesthesia. Brain was extracted, maintained in PFA for 24 hours and changed to PBS-sacarose for at least 24 hours before tissue processing. 40 mm slices were cut and stained with thionin.
Results and Discussion
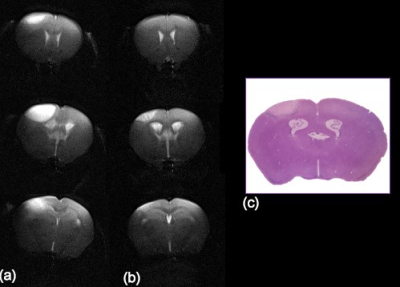
Coil implantation was successful in both animals, which did not show any sign of infection or distress after the procedure was performed.SNR of the acquired T2-weighted images was high (81 for the rat and 38 for the mouse). The high quality images allowed a clear visualization of the infarct (Fig. 2) and a longitudinal follow up of the lesion (Fig. 3).
At the acute stage, the T2-weighted images showed a hyperintense lesion affecting the cortex, from the midline to the lateral area. In the mouse (Fig. 3), at the chronic stage the images showed heterogeneous signal at the site of infarction with diminished size.
Conclusion
This work demonstrates the feasibility of using implanted wireless ICCs to image rodents in clinical scanners. The implanted coils can provide high-quality images of the brain structures and follow up of the infarct lesion in longitudinal studies.Acknowledgements
Government of Navarra Grant 0011-1365-2017-000106.References
1. Iñigo I, Isturiz J, Fernández M, et al. Imaging of stroke in rats using a clinical scanner and an inductively coupled specially designed receiver coil. ISMRM 2019, p. 1553.
2. Herrmann K, Schmidt S, Kretz A et al. Possibilities and limitations for high resolution small animal MRI on a clinical whole-body 3T scanner. Mag Reason Mater Phy. 2012; 25:233-244.
3. Graft H, Martirosian P, Schick F et al. Inductively coupled rf coils for examinations of small animals and objects in standard whole-body MR scanners. Med Phys. 2003; 30:1241-6.
4. Schnall M, Barlow C, Subramanian H, Leigh J. Wireless implanted magnetic resonance probes for in vivo NMR. J Magn Reson 1986; 68:161-167.
5. Silver X, Ni W, Mercer E, et al. In Vivo 1H magnetic resonance imaging and spectroscopy of the rat spinal cord using an inductively-coupled chronically implanted RF coil. Mag Reson Med 2001; 46:1216-1222.
Figures