4728
Lymphotropic Nanoparticle-Enhanced LNMRI for Accurate Prediction of Nodal Status in Canine Head and Neck Cancer Patients1Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2Colorado State University, Fort Collins, CO, United States, 3Radiology, University of Colorado Anschutz, Denver, CO, United States
Synopsis
The objective of this study was to develop an optimal iron oxide enhanced lymph node MRI protocol and robust image analysis using pre-and-post ferumoxytol T2 and T2* scans in canine patients with HNC. Since those pet patients are naturally prone to HNC, they can serve as a great translational platform for development of novel non-invasive imaging modalities for LN staging and metastasis and to guide anti-lymphangiogenic therapies. The results indicate that Ferumoxytol can be used as T2 negative contrast agent for non-invasive LN staging 48 hrs post-injection. SI normalization should be performed to a phantom or to the brain.
Introduction
Head and neck cancer (HNC) is a highly metastatic debilitating cancer of lip, oral cavity, pharynx, larynx, and paranasal sinuses that accounts for about 4% of all cancers in the United States (1). Approximately two-thirds of HNCs are diagnosed in advanced stage with an overall five-year survival rate below 50% (2). Clinically, comprehensive staging of malignant lymph nodes (LN) is a major prognostication of tumor metastasis, survival rates and therapy stratifications. Unfortunately, conventional anatomical MRI lacks sensitivity to diagnose LN metastasis (3), thus invasive biopsy is often required. Several previous reports have explored the possibility to utilize long-circulating iron oxide nanoparticles for non-invasive LN staging using T2-weighted MRI (4,5). Ferumoxytol is an FDA approved intravenous iron oxide formulation, used to treat iron deficiency. It has also a superb r2 relaxivity and has proven to be an excellent T2-MRI contrast, which is readily taken up by the reticuloendothelial system including normal innate lymph cells, but does not accumulate inro malignant cells (6). Therefore, the objective of this study was to develop an optimal LNMRI protocol and robust image analysis using pre-and-post ferumoxytol T2 and T2* scans in canine patients with HNC. Since those pet patients are naturally prone to HNC, they can serve as a great translational platform for development of novel non-invasive imaging modalities for LN staging and metastasis and to guide anti-lymphangiogenic therapies.Methods
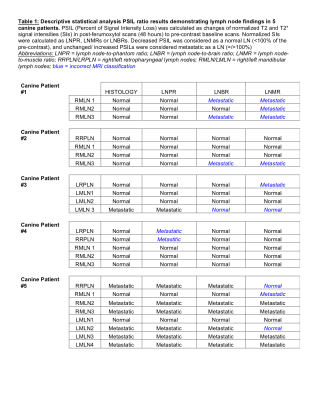
All animal protocols were approved by the CSU clinical review board and written client consents were obtained. Six canine patients with metastatic HNC were initially enrolled in this study. Patients were anesthetized, and the initial baseline imaging was performed. All images were acquired using GE HDxt 1.5 Tesla clinical scanner,16 channel MRI and GE Head/Neck/Spine Array coil. An MRI protocol based on T2* GRE, T2 FSE and pre/post gadolinium T1 sequence was obtained at the baseline. A saline vial was added within the field of view (FOV) for precise T2(*) signal intensity (SI) normalizations. Immediately after the baseline scans, the dogs were slowly infused with 7.5 mg Fe/kg ferumoxytol. The post-ferumoxytol MRI protocol was repeated at 24 and 48 hrs. ROI analysis was based on pre-and-post ferumoxytol T2(*) SIs of LNs, saline, muscles and brain (Antech Imaging Services and Horos software). All sentinel or potentially malignant LNs identified by indirect lymphography were surgically excised for histology. Descriptive statistical analysis of SI ratios LN-to-phantom (LNPR), LN-to-brain (LNBR) and LN-to-muscle (LNMR) was performed.Results
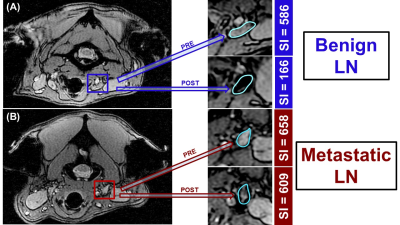
No adverse reaction to ferumoxytol was observed in the animals. From all enrolled dogs, the first male patient was re-scanned 24 hrs post-injection revealing low ferumoxytol accumulation into normal LNs, thus he was excluded from the analysis. All other patients (n=5) underwent post-ferumoxytol MRI sessions at 48 hrs. For the semi-quantitative image analysis, we normalized the T2(*) SI from each LN (bilateral retropharyngeal and several mandibular) to the SI of the saline phantom, brain and muscle. A total of 24 LNs were analyzed by MRI and histology. Histology classified 18 LNs as normal and 8 as malignant. In MRI, a decrease in normalized LN SI in post-ferumoxytol scans, evident as darkening of T2(*)-weighted images (Figure 1A), were considered “normal”. On the contrary, the post-contrast T2(*) SI of “malignant” LN demonstrated no change or an increase from the baseline, resulting in brightening of the affected LNs. (Figure 1B). The best correlation with histopathology was observed with saline phantom LNPR normalization with only 2 misclassified as false positive LN (2/24, Table 1). Brain normalization (LNBR) showed the second-best correlation with histopathology (four false positive, two false negative, 6/24), while muscle normalization (LNMR) had the worst correlation rate (9/24).Discussion
These results indicate that Ferumoxytol can be used as T2 negative contrast agent for non-invasive LN staging 48 hrs post-injection. The absence of T2(*) contrast changes by ferumoxytol confirms the presence of malignant HNC cells (which do not uptake iron oxide) and altered parenchymal characteristics in malignant LNs (which make them impenetrable to ferumoxytol). Best clinically applicable SI normalization for LN in HNC patients should be performed to a phantom (LNPR, if present) or to the brain (LNBR), but not to the muscle.Acknowledgements
No acknowledgement found.References
[1] Tang, Xiaoxia et al. "Efficacy And Safety Of Gefitinib In Patients With Advanced Head And Neck Squamous Cell Carcinoma: A Meta-Analysis Of Randomized Controlled Trials". Journal Of Oncology, vol 2019, pp.1-9.
[2] Vogelzang, N.J., et al. “Clinical cancer advances 2011: Annual Report on Progress Against Cancer from the American Society of Clinical Oncology”. Journal of Clinical Oncology, 2012, pp 88-109.
[3] Linden, Hannah M., and Farrokh Dehdashti. "Novel Methods And Tracers For Breast Cancer Imaging”. Seminars In Nuclear Medicine, vol 43, no. 4, 2013, pp. 324-329.
[4] Ross, Robert W. et al. "Lymphotropic Nanoparticle-Enhanced Magnetic Resonance Imaging (LNMRI) Identifies Occult Lymph Node Metastases In Prostate Cancer Patients Prior To Salvage Radiation Therapy”. Clinical Imaging, vol 33, no. 4, 2009, pp. 301-305.
[5] McDermott, Shaunagh et al. "Accurate Prediction Of Nodal Status In Preoperative Patients With Pancreatic Ductal Adenocarcinoma Using Next-Gen Nanoparticle".Translational Oncology, vol 6, no.6, 2013, pp.670-675.
[6] Thurman, Joshua M., and Natalie J. Serkova. "Nanosized Contrast Agents To Noninvasively Detect Kidney Inflammation By Magnetic Resonance Imaging". Advances In Chronic Kidney Disease, vol 20, no. 6, 2013, pp. 488-499.