4725
13C-MRI Detects Increased Lactate Flux in HCC Following Hepatic Thermal Ablation and Correlates with PFKFB3 Expression1Radiology, Beth Israel Deaconess Medical Center, Boston, MA, United States
Synopsis
Thermal ablation (TA) is commonly used to treat early hepatocellular carcinoma (HCC) and liver metastases. However, there is increasing clinical and experimental evidence that TA may sometimes induce more aggressive tumor biology within and outside the liver. We used hyperpolarized 13C MRI to detect increased HCC lactate flux following adjacent normal liver radiofrequency ablation in a rat model. This increased flux correlated with an increase in tumor expression of PFKFB3, a known modulator of glycolysis. PFKFB3 may be a potential therapeutic target and hyperpolarized 13C MRI may be used to track or predict treatment response.
Introduction
Thermal ablation (TA) is an accepted therapy for early HCC1 and colorectal and breast cancer liver metastases.2 However, suboptimal TA for HCC is a risk factor for early diffuse recurrence.3 This recurrence can be associated with a reduction in overall survival.4 This effect has been attributed to hypoxic response in periablative tissue mediated by hypoxia-inducible transcription factor-1 (HIF-1).5 Malignant transformation of normal cells leads to increased glucose uptake and lactate formation even under normoxic conditions.6 Hyperpolarized 13C MRI (h13C MRI) enables real-time high-resolution imaging of the uptake and metabolism of tracer molecules such as pyruvate, lactate, and alanine and may be used as a potential predictor for therapeutic response in HCC.7,8 We identified two potential metabolic regulators and therapeutic targets with a direct link to lactate flux which may be monitored with h13C MRI. The gene PFKFB3 encodes for inducible 6-phosphofructo-2-kinase (iPFK2), enhancing the production F2,6P2, which consequently activates PK1 and stimulates glycolysis.9 PFKFB3 has been suggested to play a crucial role in many types of tumor cells as well as various cells within the tumor microenvironment.10 Hexokinase 3 (HK3), a key catalyst in the first committed steps in glucose metabolism, is also a promotor involved in epithelial–mesenchymal transition process.11 Here we used a rat model of HCC to measure tumor lactate flux in response to thermal ablation of adjacent normal hepatic tissue, simulating local treatment of a distant lesion. We then correlated lactate flux as measured by h13C MRI to expression levels of metabolic signaling factors including PFKFB3 and HK3.Methods
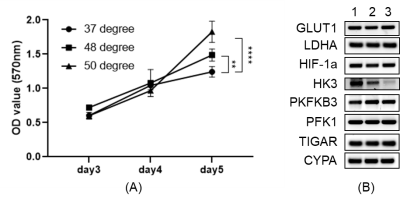
In vitro experimentsN1S1 cells (rat hepatoma cell line, ATCC) were exposed to different temperatures (37°C, 48°C, 50°C, and 55°C) for 5min, then seed back to culture tank. After 3 days, the cell viability of different groups was detected by apoptosis kit. MTT assay was performed on samples from the harvested tumors to assess cell proliferation, and RT-qPCR was performed for assessment of mRNA expression of glycolysis-related genes.
In vivo experiments
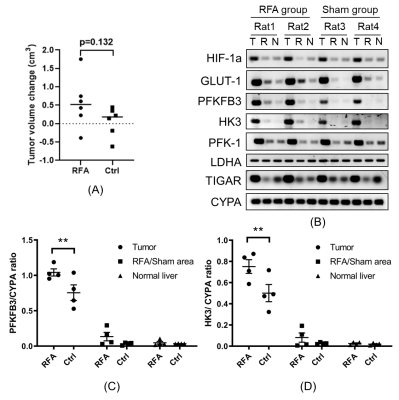
2x106 N1S1 cells were implanted into the left lobe of Fischer 344 rats. When implanted tumors reached 10 mm as measured by ultrasound, the rats were randomized into a sham (control) group versus a RFA group. RFA was performed at 70 ℃ for 5 min on normal tissue within the right hepatic lobe. In the sham group all of the steps of RFA were performed except the treatment probe was not energized. Hyperpolarized 13C MRI was performed prior to treatment and 72 hours post-treatment, and tumors were then harvested.
Hyperpolarized 13C pyruvate solution was prepared by dynamic nuclear polarization (DNP) using a commercial polarizer (Hypersense, Oxford Instruments). [1-13C] pyruvic acid was combined with 15mM OX063 radical (GE Healthcare, London UK) and 1mM gadoteridol (ProHance, Bracco, Milan Italy) and polarized for 40 minutes or more at 1.4K and 100mW microwave power. The hyperpolarized material was then dissolved in saline containing 50mM TRIS and 125mg/l EDTA and adjusted to physiological pH with sodium hydroxide to obtain a 96mM hyperpolarized pyruvate solution. 2.5ml were administered via tail vein. Imaging was performed on a 9.4 T magnet (Biospec, Bruker). 13C data were acquired in the axial plane across the tumor using an echo planar spectroscopic imaging (EPSI) sequence with FOV of 5 cm, 4° tip angle, 16x16 matrix, 10mm slice thickness, 512 spectral points, and 4kHz spectral width. 64 repetitions were acquired with 2s temporal resolution.
Results
In vitro heat-treatment of N1S1 cell line demonstrated 20%-90% cell survival at 40℃-50℃ and no survival at 55℃. Cellular proliferation rate was significantly higher in the heat-treated groups (Fig. 1A). mRNA expression of PFKFB3 was slightly increased after thermal treatment, though not statistically significant, while HK3 expression was significantly decreased (Fig. 1B). In the in vivo study, tumors in the RFA arm and control arms did not demonstrate a significant difference in growth (Fig. 2A). There was increased expression of PFKFB3 and HK3 in tumors relative to liver tissue, and tumors in the RFA group demonstrated greater PFKFB3 and HK3 expression as compared to the control group (Fig. 2B-D). Hyperpolarized 13C MRI imaging demonstrated relatively increased tumor 13C lactate flux in RFA arm relative to the control group (Fig. 3B), while background liver lactate flux remains unchanged (Fig. 3C).Discussion
In vivo h13C MRI demonstrated increased lactate flux within HCC tumors following thermal ablation of adjacent normal liver, which correlated with an increase in PFKFB3 and HK3 expression, both key regulators in glycolysis. The PFKFB3 results were concordant with our prior in vitro experiments, while H3K expression demonstrated an opposite trend. Expression levels of PFKFB3 have been found to be higher in malignancies than in normal tissues,12 attributed to loss of glycolytic control and accelerating neoplastic activity.10 Our data shows that PFKFB3 may be an attractive treatment target for HCC, and hyperpolarized 13C MRI may be a noninvasive technique well-suited to monitor treatment response.Conclusion
Lactate flux within a rat HCC tumor model was increased following adjacent normal liver RFA, correlating with increased PFKFB3 expression.Acknowledgements
We are grateful for support from the RSNA Research Scholar Grant and the society of Abdominal Imaging Dodds Award.References
1. Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249(1):20-5.
2. de Baère T, Aupérin A, Deschamps F. et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann. Oncol. 2015;26,987–991.
3. Lee HY, Rhim H, Lee MW. et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23(1):190-7.
4. Ng KK, Poon RT, Lo CM. et al. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008; 12(1):183-91.
5. Singh D, Arora R, Kaur P. et al. Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci. 2017;13;7:62.
6. Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: A Metabolic Key Player in Cancer. Cancer Res. 2011;71(22):6921-5.
7. Zhang, H. The Potential of Hyperpolarized 13C MRI in Assessing Signaling Pathways in Cancer. Acad Radiol. 2014;21(2):215-22.
8. Albers MJ, Bok R, Chen AP. et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008;68(20):8607-15.
9. Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011;76:211-6.
10. Shi L, Pan H, Liu Z. et al. Roles of PFKFB3 in cancer. Signal Transduct Target Ther. 2017;2:17044.
11. Pudova EA, Kudryavtseva AV, Fedorova MS. et al. HK3 overexpression associated with epithelial-mesenchymal transition in colorectal cancer. BMC Genomics. 2018;19(Suppl 3):113.
12. Lu L, Chen Y, Zhu Y. The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget. 2017;8(37):62793-62802.
Figures