4718
Microvascular Architecture Changes in the Brain of an Alzheimer’s disease Mouse1Radiology, Hallym University medical center, Hwasung, Kyung-gi-Do, Republic of Korea, 2Department of physics, Kyung Hee University, Seoul, Republic of Korea, 3Department of Biomedical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Republic of Korea, 4Department of Physics and Research Institute for Basic Sciences, Graduate School, Kyung Hee University, Seoul, Republic of Korea, 5Department of Neurology, Kyung Hee University Hospital, Seoul, Republic of Korea, 6Department of Neurology, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea, 7Department of Radiology, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea
Synopsis
To characterize and evaluate microvascular architectures presented by brain microvascular indices obtained with a 7T animal MRI system in the transgenic (Tg) AD-model mice and the non-Tg mice using monocrystalline iron oxide nanoparticle (MION) contrast agent, seven non-transgenic (Tg) mice and ten 5xFAD Tg mice were scanned to measure the R2 and R2* relaxation rates before and after injection of MION contrast agent. ΔR2, ΔR2*, BVf, mVD, VSI, and MvWI were greater in the Tg mouse group than in the non-Tg mouse group. ADC and mean vessel density Q were not significantly different between the two groups.
Introduction
Mechanisms of vascular pathology in Alzheimer’s disease (AD) include changes in vessel morphology, impairment of vascular reactivity, vascular stenosis, neurovascular uncoupling, and blood-brain barrier (BBB) dysfunction. The microvascular structures in AD by autopsy demonstrated the decreased smooth muscle actin, resulting increase of wall thickness of cerebral artery caused by accumulation of Aβ and/or the protein tau (1). In AD, cerebral amyloid angiopathy (CAA) characterized by depositions of Aβ in the cortical and leptomeningeal vessel walls plays a major role (2) and is responsible for brain hypoperfusion and dysfunction. Therefore, the study of microvasculature is important for the comprehension of pathologic mechanism of AD. The microvascular MR imaging is a relatively new imaging method based on the measurement of the changes ∆R2 and ∆R2* in transverse relaxation rate constants before and after injection of an intravascular contrast agent (3). Although the microvascular MR techniques were applied in tumors, stroke, and dementia (4), few studies have been performed to investigate the microvascular injuries of the AD brain. Therefore, the objective of this study was to characterize and evaluate microvascular architectures presented by brain microvascular indices obtained with a 7T animal MRI system in the transgenic (Tg) AD-model mice and the non-Tg mice using monocrystalline iron oxide nanoparticle (MION) contrast agent.Methods
Seven non-transgenic (Tg) mice and ten 5xFAD Tg mice were scanned using 7T animal MRI system (Bruker Biospec GmbH, Ettlingen, Germany) to measure the R2 and R2* relaxation rates before and after injection of the monocrystalline iron oxide nanoparticle (MION) contrast agent. To measure the R2 relaxation rates before and after injection of the contrast agent, 2D multi-slice multi-echo spin-echo (MESE) images were acquired using the following parameters: TR = 1300 ms, TEs = 40, 80, 120, and 160 ms. To measure R2* relaxation rates before and after injection of the contrast agent, 2D multi-slice multi-echo gradient-echo (MEGE) images were acquired with following parameters: TR = 1500 ms, TEs = 1.81, 5.81, 9.81, 13.81, 17.81, 21.81, 25.81, 29.81, 33.81, and 37.81 ms. Finally, to map apparent diffusion coefficient (ADC), diffusion-weighted images (DWI) was acquired with a spin-echo echo planar imaging (EPI) pulse sequence. The microvascular indices of the vessel size index (VSI) (3), the mean vessel diameter (mVD) (3), the mean vessel density (Q) (3), the mean vessel-weighted image (MvWI) (5), and blood volume fraction (BVf) (3) were calculated using ΔR2* and ΔR2. To compare all maps between the non-Tg and Tg groups, the voxel-based independent t-test was used. Month was used as a covariate. Those voxel-based analyses were performed to select brain areas to obtain the microvascular index values for regions-of-interests (ROIs)-based analyses.Results
In the voxel-based comparisons, ΔR2 was greater in the Tg mouse group than in the non-Tg mouse group at the somatosensory cortex. ΔR2* was also greater in the Tg group than in the non-Tg group at the somatosensory cortex, cerebral cortex, and motor cortex. BVf was shown in similar result as the ΔR2*. mVD, VSI, and MvWI were also greater in the Tg group than in the non-Tg group. ADC and mean vessel density Q were not significantly different between the two groups.In the ROI-based analysis, ΔR2* and BVf were significantly different between the two groups. mVD and VSI were significantly different between the two groups. MvWI was significantly different between the two groups. ΔR2 and Q were not significantly different between the two groups for all ROIs. Most of ROIs defined by the result of the voxel-based analysis were significantly different between the two groups, but most of ROIs defined by the mouse brain atlas were not significantly different. Microvascular indices were significantly increased in the Tg mouse compared with the non-Tg mouse in the right somatosensory cortex, but not in the hippocampus.
Conclusion
We investigated cerebral microvascular injuries in the AD-model mouse brain using MRI techniques. We found that microvascular indices including VSI were significantly increased in the Tg mouse group compared with the non-Tg mouse group in the somatosensory cortex area, reflecting the vascular morphologic pathology of the Tg mice, which may be related to vascular pathology or damages of the neurovascular unit in AD. Based on our result, the investigation of microvascular structure in the human AD brain could be whirthwhile using a high-field MRI system for improved early diagnosis of and treatment monitoring in AD.Acknowledgements
The research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03930720, GHJ) and supported by the grant of the Convergence of Conventional Medicine and Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HI16C2352, GHJ).References
1) Postupna, N., Keene, C.D., Crane, P.K., Gonzalez-Cuyar, L.F., Sonnen, J.A., Hewitt, J., Rice, S., Howard, K., Montine, K.S., Larson, E.B., Montine, T.J., 2015. Cerebral cortical Abeta42 and PHF-tau in 325 consecutive brain autopsies stratified by diagnosis, location, and APOE. J Neuropathol Exp Neurol 74, 100-109.
2) Patel, N.S., Quadros, A., Brem, S., Wotoczek-Obadia, M., Mathura, V.S., Laporte, V., Mullan, M., Paris, D., 2008. Potent anti-angiogenic motifs within the Alzheimer beta-amyloid peptide. Amyloid 15, 5-19.
3) Tropres, I., Pannetier, N., Grand, S., Lemasson, B., Moisan, A., Peoc'h, M., Remy, C., Barbier, E.L., 2015. Imaging the microvessel caliber and density: Principles and applications of microvascular MRI. Magn Reson Med 73, 325-341.
4) Zerbi, V., Jansen, D., Dederen, P.J., Veltien, A., Hamans, B., Liu, Y., Heerschap, A., Kiliaan, A.J., 2013. Microvascular cerebral blood volume changes in aging APP(swe)/PS1(dE9) AD mouse model: a voxel-wise approach. Brain Struct Funct 218, 1085-1098.
5) Jung, H.S., Jin, S.H., Cho, J.H., Han, S.H., Lee, D.K., Cho, H., 2016. UTE-DeltaR2 -DeltaR2 * combined MR whole-brain angiogram using dual-contrast superparamagnetic iron oxide nanoparticles. NMR Biomed 29, 690-701.
Figures
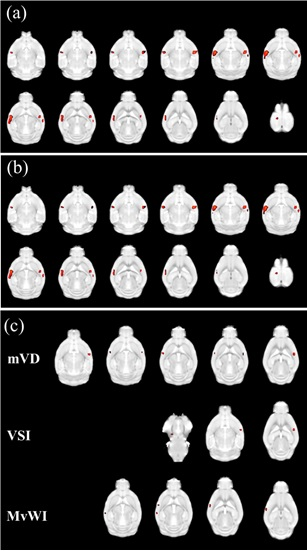
Figure 1. Result maps of the voxel-based comparisons between the non-Tg and Tg groups of ΔR2* (a), blood volume fraction (BVf) (b), and mean vessel diameter (mVD), vessel size index (VSI), and microvessel-weighted image (MvWI) (c).
Red color indicates increased microvascular indices in the Tg mouse group compared with in the non-Tg mouse group. There were no areas shown in greater indices in the non-Tg mouse group than in the Tg group.
The significant level was used with the p=0.001 without correcting the multiple comparisons and with the threshold of 30 contiguous voxels.