4712
Glymphatic monitoring in awake state mouse brain with DCE-MRI imaging1Division of Glial Disease and Therapeutics, University of Copenhagen, Center for Translational Neuromedicine, Copenhagen, Denmark, 2Division of Glial Disease and Therapeutics, University of Rochester Medical Center, Center for Translational Neuromedicine, Rochester, NY, United States, 3Faculty of Health and Medical Sciences, University of Copenhagen, Panum NMR Core Facility, Copenhagen, Denmark
Synopsis
The glymphatic pathway is a novel pathway for interstitial solute clearance. Previous reports note that glymphatic patterns differ according to brain activity. Due to the motion-induced image blurring effects, DCE-MRI in the awake animal is difficult. We aimed to determine the feasibility of FISP-based fast DCE-MRI to assess the glymphatic pathway in awake mice. Head-fixed mice were habituated to restraint and received a cisterna magna cannulation. Awake DCE-MRI was performed 24 hours post surgery. Dynamic tracer distribution maps were calculated, and tracer distribution was measured in each brain segmentation. We successfully obtain DCE-MRI and demonstrate glymphatic dynamics in awake mice.
Introduction
The glymphatic pathway is a novel pathway for interstitial solute clearance. Previous literature has reported that the state of brain activity (i.e. the sleep-wake cycle) controls glymphatic activity, with CSF influx being higher in sleeping or anesthetized than in awake mice 1. All prior experimental studies of the sleep-wake regulation of the glymphatic system have been based on optical techniques including two-photon or macroscopic imaging. While these microscope approaches have their advantages, they only allow pinhole imaging of small field or the field of view is limited to the cortical surface. Often cranial window are prepared 1,2. The use of a less-invasive MRI method in awake rodents would be beneficial for future studies of glymphatic kinetics. The time series of T1-weighted (T1W) 2D/3D fast low angle shot (FLASH) sequence, acquired during and after paramagnetic contrast delivery into the CSF space, have previously been used to assess glymphatic function. However these conventional T1W dynamic contrast enhance MRI (DCE-MRI) method needs a couple of minutes for one frame, and this low temporal resolution increases the risk of motion-induced image blurring in awake mice. Fast image sequence, including 3D Fast Imaging with Steady-state Free Precession (3D-FISP) can potentially solve this issue.Purpose
We aimed to determine the feasibility of FISP-based DCE-MRI for quantifying glymphatic CSF transport in awake mice.Methods
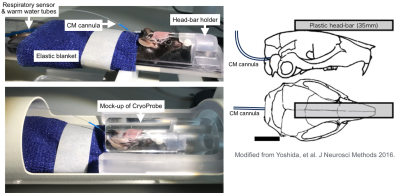
Headplates were surgically implanted in all mice 5-7 days before the scans using a technique previously reported 3. Each animal was habituated to restraint in a mock MR mounting device for two days before scanning. The schedule was such that there were three sessions (morning, noon, and afternoon) per day, which gradually increased in habituation time, ranging from 5-60 minutes. A permanent cannula was implanted into the cisterna magna (CM) under isoflurane after completion of the training. Awake DCE-MRI was scheduled to 24 hours after the surgery. MRI was performed using a 9.4 Tesla preclinical scanner (BioSpec 94/30 USR, Bruker BioSpin, Ettlingen, Germany) equipped with a cryogenically-cooled quadrature-resonator (CryoProbe, Bruker BioSpin, Ettlingen, Germany). Mice were placed in the custom-made MR-compatible holder in a prone position and the mouse head restrained using the headplate (Figure1). Body temperature was maintained at 37 °C and monitored along with the breathing rate by a remote monitoring system (SA Instruments, NY, USA). T2-weighted rapid acquisition and relaxation enhancement scans (2D RARE: TR/TE: 9000/20ms, Matrix 192 × 128, FOV 19.2 mm × 12.8 mm, NEX 4, 76 sagittal slices, slice thickness = 0.2 mm) TOF-MRA (2D Flow-compensated FLASH: TR/TE: 15/3ms, Matrix 192 × 128, FOV 19.2 mm × 12.8 mm, NEX 4, 150 sagittal slices, slice thickness = 0.1 mm) and CSF cisternography with 3D-TrueFISP sequence (TR/TE 4.2/2.1 ms, Scan TR 848 ms, FA=50°, Matrix 256 × 192 × 192, FOV 19.2 × 14.4 × 14.4 mm, NEX 4) were performed. The contrast agent, gadobutrol (Gadovist, Bayer Pharma AG, Leverkusen, Germany) was infused into CM (12.5 mM gadobutrol at a constant rate of 1.5 µL/min for 8 min) followed by continuous MRI every 30 seconds for 60 min using the post excitation refocussed 3D Fast Imaging with Steady-state Free Precession (3D-FISP) sequence (TR/TE 3.26/1.63 ms, FA=15°, Single-shot, ScanTR=325.75ms, Matrix 192 × 128 × 128, FOV 19.2 × 12.8 × 12.8 mm, Nex 1). After the pre-processing, dynamic tracer distribution maps were calculated, and brain uptake and clearance were measured in each brain segmentation.Results
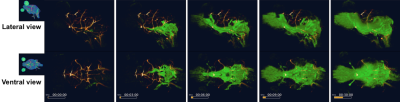
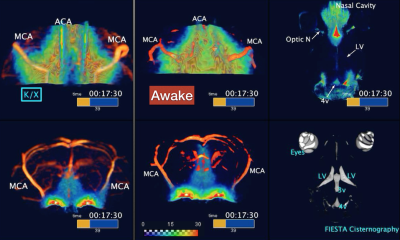
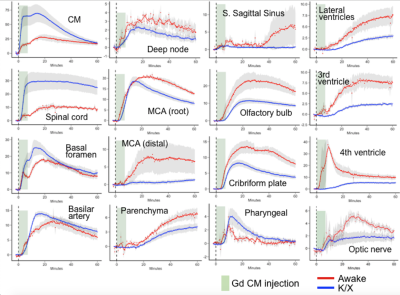
FISP-based DCE-MRI successfully demonstrates a distribution of CSF tracer in the awake mouse brain without severe motion-induced blurring effects (Figure2). CSF tracers distributed along the circle of Willis, the perivascular spaces, and entered the brain parenchyma. We also compared the tracer distribution differences between awake state and under ketamine-xylazine anesthesia. Significant differences in CSF transport dynamics was noted when comparing awake and anesthetized mice (Figure3,4).Discussion
Because of the longer time required while conducting MR imaging sequences, compared to other imaging modalities, like fluorescence imaging, this technique has traditionally been more sensitive to physiological motions of the subject. Shorten the scan time with an advantage of 3D-FISP fast image sequence reduce these motion-induced blurring effects, in turn enabling high resolution imaging of awake subjects. Although we need to consider stress induced by restraining the awake mice, habituation to head restraining have in prior studies been shown to minimize stress. Awake glymphatic MR imaging is expected to provide new insight into the mechanisms of interstitial solute influx and clearance mechanisms.Conclusion
We successfully obtain DCE-MRI and demonstrate glymphatic dynamics in awake state mice. Motion-induced artifact were well controlled by fast FISP-based DCE acquisition. This awake DCE-MRI method provides a novel way to monitor certain CSF dynamics in different states of the brain activity and provides a benefit not only for studies of rodent glymphatic but also for translational research in humans.Acknowledgements
No acknowledgement found.References
1. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–377.
2. Desjardins M, Kılıç K, Thunemann M, et al. Awake Mouse Imaging: From Two-Photon Microscopy to Blood Oxygen Level–Dependent Functional Magnetic Resonance Imaging. Biol Psychiatry 2019;4(6):533–542.
3. Yoshida K, Mimura Y, Ishihara R, et al. Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe. J Neurosci Methods 2016;274:38–48.
Figures