4707
Longitudinal voxel-based analysis in Alzheimer’s disease transgenic marmosets1Central Institute for Experimental Animals, Kawasaki, Japan, 2Department of Physiology, Keio University School of Medicine, Tokyo, Japan, 3Department of Radiological Science, Tokyo Metropolitan University, Tokyo, Japan
Synopsis
This study investigated whether MRI could detect brain abnormalities in transgenic marmoset models of Alzheimer’s disease (AD). Magnetization transfer (MT) contrast-MRI have a potential to detect the presence of amyloid plaques, which could be present in the early stage of the diseases. Voxel-based analysis was conducted to explore whether any difference existed between AD models and healthy marmosets (young-to-middle-aged adults). Significant increase of MT-ratios imaging was observed in posterior cingulate cortex whereas decrease was observed in unilateral inferior temporal cortex. This result supported MT-imaging was sensitive to early abnormalities. Continuous evaluation until old age is worthwhile to be clinically relevant.
INTRODUCTION
Transgenic techniques have been the most commonly used for the animal models of Alzheimer’s disease (AD). The reasons included evaluating brain abnormalities before symptoms appeared, which allowed providing knowledge which human studies were difficult to reach. The studies using AD disease transgenic mice reported Magnetization Transfer Contrast MRI (MTC-MRI) were sensitive to track amyloid plaque, which might be already present in the early stage of disease12. However, the findings were inconsistent in that MT-Ratios decreased in human studies3 bur increased in mouse studies. It would be possibly due to the MT-Ratios increase in early stage of AD, but few studies examined whether the species gap between rodents and humans might influence its discrepancy. To address it, this study applied AD transgenic models of (?) the common marmosets (marmosets), non-human primates. Longitudinal MTC-MRI was performed to the marmosets AD models at the age from young-middle-aged adult to detect brain abnormalities in early onset.METHODS
<Marmosets>We produced the transgenic marmosets overexpressing the Swedish mutant of human APP, which we evaluated as the AD model (AD model, N=2, both were female). MRI was performed at the age of 3.5 years. We repeated the scan per year until 5.5/6.5 years old, 3 times in total (i.e., 3.5, 4.5, and 5.5 years old). Control MRI data were acquired from adult healthy marmosets (N=30) ranged from 2 to11 years old (mean age = 5.7 years old, female N=15, male N=15). In general, marmosets reach adulthood by 2 years old and 8 years old is considered as aged4.
<Image acquisition> T1-weighted images (T1WI) and MTC were acquired using 7.0 T Biospec 70/16 scanner (Bruker BioSpin:Ettlingen, Germany) with following parameters (T1WI: repetition time/TR, 6000 millisecond/ms; echo time/TE, 35 ms; inversionTime ; 1300 ms. image matrix 192 x 192 x 120. MT–Ratios: FLASH with/without an off-resonance frequency set bandwidth (bandwidth: 300 Hz, off-set frequency: 1500 Hz) TR: 2600 ms, TE: 2.86 ms, flip angle 70°, image matrix 128 x 128 x 54).
<Image analysis> For conducting voxel-based analysis, T1WI and MT-Ratios images were preprocessed using statistical parametric mapping (SPM12) running on Matlab. After preprocessing using DARTEL5, a two-sample t-test was performed to compare the difference between disease models and control. We repeated the statistical analysis as we acquired the AD model data per year using the same control data.
RESULTS
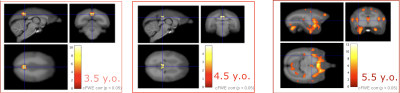
Voxel-based morphometry (VBM) using T1WI showed no significant decrease in volume at any time point, whereas significant volume increase was observed at 3.5 years old at the right hippocampus, which was not observed thereafter. On the other hand, significant MT-Ratio decrease was observed for the one subject in the left side of the inferior-temporal cortex at any time-points (3.5,4.5,5.5 years old). There was also significant increase in the part of precuneus/posterior cingulate cortex until 4.5 years old. Although significant increase was not observed in precuneus/posterior cingulate cortex at the age of 5.5 years, the significant increase was observed in multiple regions such as the primary visual cortex and hippocampus.DISCUSSION
Voxel-based analysis with MT-Ratio detected the regions showing significant differences existed between control and AD disease models. The finding was relatively similar to the previous report in mouse: MT-Ratio increase in the retrosplenial cortex, while our analysis showed MT-Ratio increase in the left side of the posterior cingulate cortex. The correlation between MT-Ratio and immunohistochemistry indicated the region might contain high amyloid load1. Our analysis also showed MT-Ratios decrease was significant in the left side of inferior-temporal cortex. Precious mouse study, in contrast, showed MT-Ratio decreased in white matter, the corpus callosum splenium, but not at the gray matter2. The marmoset result could be close to human in that human studies found decreased MT-Ratio in gray matter. The difference lay in that the decreased MT-Ratio was observed in the hippocampus and the lateral occipital cortex. Given AD patients were not at the stage of early onset, it would be necessary to continue data acquisition to compare with marmosets. This study was limited in that the number of AD model was small due to a limited number can be produced. Nonetheless, MRI evaluation using marmoset AD model is necessary to investigate to get the result the previous closer to the human studies. Further, the correlation with immunohistochemistry would be worthwhile to valid the results and to reveal the pathological profile of these models.CONCLUSION
This study using AD model marmosets supported previous reports that MTC-MRI can be the sensitive method to detect brain abnormalities before the symptom appears. In this study, we took the exploratory approach to examine whether abnormal regions existed in AD model. Detailed analysis of detected abnormal regions would be coming tasks to examine which aspect might be normal by use of different measurements such as resting-state fMRI.Acknowledgements
This research is supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies(Brain/MINDS) from Japan Agency for Medical Research and development, AMED.References
1. Bigot C, Vanhoutte G, Verhoye M, et al., Magnetization transfer contrast imaging reveals amyloid pathology in Alzheimer's disease transgenic mice. Neuroimage. 2014;87:111-119.
2. Praet J, Bigot C, Orije J, et al., Magnetization transfer contrast imaging detects early white matter changes in the APP/PS1 amyloidosis mouse model. NeuroImage Clin. 2016,12: 85–92.
3. Giulietti G, Bozzli M, Figura V, et al., Quantitative magnetization transfer provides information complementary to grey matter atrophy in Alzheimer's disease brains. Neuroimage. 2012;59:1114-1122.
4. Schultz-Darken N, Braun MK, Emborg, EM. Neurobehavioral Development of Common Marmoset Monkeys. Dev. Psychobiol. 2016;58: 141-158.
5. Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007; 38: 95–113.
Figures
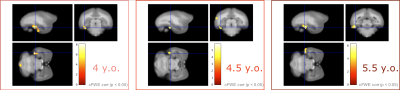
MRI images (sagittal, upper left; coronal, upper right; axial, lower left) show distribution of decreased MT-Ratio in AD model. Color scale bar (lower right) in voxel-wise statistics indicates t score, and significant areas were superimposed on the T1-weighted template brain.
Abbreviations: y.o = year old, cFWE corr = family-wise corrected, cluster level inference