4703
Replacing CT with high-resolution MRI for 3D pediatric cranial bone imaging1Plastic and Reconstructive Surgery, Washington University in St. Louis, Saint Louis, MO, United States, 2Radiology, Washington University in St. Louis, Saint Louis, MO, United States, 3Surgery, Washington University in St. Louis, Saint Louis, MO, United States, 4Neurosurgery, Washington University in St. Louis, Saint Louis, MO, United States
Synopsis
Ionizing radiation from computed tomography (CT) imaging increases the risk of cancer. Patients with craniosynostosis often undergo repeated head CT scans, exacerbating the cumulative risk. Magnetic Resonance Imaging (MRI) has the potential to be a radiation-free safe alternative. Previously proposed methods in this area are not widely utilized because of suboptimal osseous/soft tissue contrast, vulnerability to motion, and the need for manual post-processing. In this study, we propose a high-resolution radial MRI protocol with improved tissue contrast and less sensitivity to motion. Moreover, we seek to evaluate its feasibility by using a blinded clinical evaluation.
INTRODUCTION
Ionizing radiation from computed tomography (CT) imaging increases the risk of cancer. Children under 5 years of age are at greatest risk [1–6]. Patients with craniosynostosis often undergo repeated head CT scans, exacerbating the cumulative risk.Magnetic Resonance Imaging (MRI) has the potential to be a safe alternative to CT because MRI does not expose patients to ionizing radiation. Eley et al. [7,8] proposed a “Black Bone” protocol that results in dark signal intensities in the osseous tissue, enabling the extraction of skull after an inversion of the image intensities. However, Black Bone methods are not widely utilized because of (1) suboptimal osseous/soft tissue contrast, (2) motion artifacts that often require patient sedation, and (3) the need for manual, operator-dependent post-processing. As a result, the capability of MRI to produce CT-like 3D-reconstructed images of the cranium has been very limited [9]. In this pilot study, we propose to develop a high-resolution radial MRI protocol in order to improve osseous/soft tissue contrast and to reduce sensitivity to motion. Moreover, we seek to evaluate its feasibility in pediatric patients with craniosynostosis and possibly other cranial malformation, by using a blinded clinical evaluation. This study has the potential to provide the craniofacial community an alternative imaging method that avoids the risks of radiation exposure.
METHODS
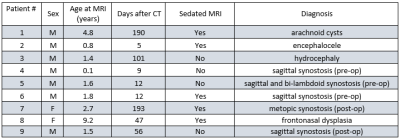
Upon Institutional Review Board approval, informed consent was obtained from the parent(s) of all participating children. Figure 1 lists the patient statistics. A healthy subject was also scanned to compare the proposed method with the Black Bone protocol.MRI images were acquired using a 3T Prisma, a 3T VIDA, and a 3T Biograph mMR scanner (Siemens Healthineers, Erlangen, Germany) with either a 32-channel or a 20-channel head coil. A Fast Low-Angle Shot (FLASH) Golden-Angle 3D stack-of-stars radial VIBE sequence (GA-VIBE) [10] was used to acquire images. A radial sequence was preferred here since it is more robust to motion. The imaging parameters were as follows: TR/TE = 4.84ms/2.47 ms, Bandwidth = 410 Hz/pixel, 224 slices per slab, transverse orientation, Flip angle = 3°, Acquisition matrix = 320 × 320, Voxel size 0.6 x 0.6 x 0.8 mm and number of radial lines = 400 for a scan duration of 5 minutes and 4 seconds. The protocol was designed to maximize the image contrast between bone and all non-osseous tissues by choosing an in-phase TE value and a small flip angle (3°) to increase proton density weighting.
The CT scans followed standard clinical pediatric methods. A multi-slice Siemens SOMATOM Definition Flash or Force CT scanner (Siemens Medical Systems, Inc., Iselin, NJ) was used with slice thickness ranging from 0.6 mm to 1mm and pixel spacing ranging from 0.31 x 0.31mm to 0.39 x 0.39 mm.
MR and CT images were manually processed in 3DSlicer [11]. First, MR images were registered to CT images for each participant using a rigid-body plus scaling transformation. MR scans were then processed with bias correction (using N4iTK algorithm), masking, intensity inversion, recursive Gaussian filtering and volume rendering to yield 3D images of the skull. CT scan rendering follows standard clinical pediatric methods and employs the preset bone intensity threshold. Rendered images of the reconstructions in different views were then captured at 15° increments in horizontal and vertical rotations for a total of 48 images (24 horizontal and 24 vertical) per patient scan. The screenshots for each MRI and CT scan were later compiled into a slide deck for review.
A craniofacial plastic surgeon (KBP), a pediatric neurosurgeon (MDS), and a neuroradiologist (MSG) compared the 3D-reconstructed images from MRI data to the gold-standard CT-based images. The reviewers were blinded to scan modality and patient information. They independently assessed the anatomic presence or absence (open, partially open, or closed) of the six cranial sutures.
RESULTS
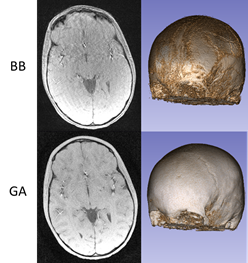
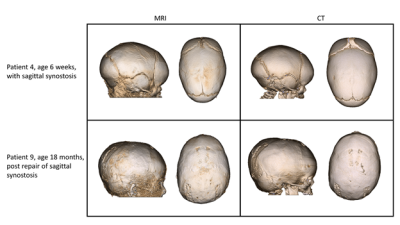
Figure 2 exhibits the robustness of the radial GA-VIBE sequence to motion, in comparison with the Cartesian Black Bone sequence. Both sequences were run back-to-back and the Black Bone sequence seems more vulnerable to motion.Figure 3 demonstrates 3D renderings for two patients. The resemblance of the MR-based renderings to the CT-based ones is promising.
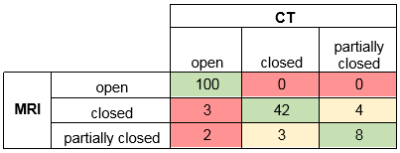
Since there were 3 reviewers, 9 patients and 6 sutures per patient, 162 assessments were made per imaging modality. As for visibility, reviewers reported clear imaging of the sutures in 159 cases for CT and in 157 cases for MR. The remaining sutures were marked “partially visible”. As for the sensitivity and the specificity of MRI to suture closure, Figure 4 compares the classification of the sutures for both modalities. The sensitivity of MRI to suture closure was 100% (45/45) while the specificity was 95% (100/105).
DISCUSSION
The GA-VIBE sequence is high-resolution, robust to motion, and has competent tissue contrast to allow for the extraction of skull without much difficulty. This method is 100% sensitive and 95% specific to suture closure, which is critical for clinical diagnosis. The main advantage is saving children from radiation, while the main disadvantage is the acquisition time compared to CT.CONCLUSION
The GA-VIBE method was shown to be capable of providing clinically acceptable 3D-reconstructed cranial images. Future directions include reducing the scan time, applying motion correction, and automation of post processing for clinical utility.Acknowledgements
No acknowledgement found.References
1. Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–707.
2. Brenner DJ, Elliston CD, Hall EJ, et al. Estimates of the cancer risks from pediatric CT radiation are not merely theoretical: comment on “point/counterpoint: in x-ray computed tomography, technique factors should be selected appropriate to patient size. against the proposition.” Med Phys. 2001;28(11):2387–2388.
3. Parker L. COMPUTED TOMOGRAPHY SCANNING IN CHILDREN: Radiation Risks. Pediatric Hematology and Oncology. 2001;18(5):307–308.
4. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505.
5. Smyth MD, Narayan P, Tubbs RS, et al. Cumulative diagnostic radiation exposure in children with ventriculoperitoneal shunts: a review. Childs Nerv Syst. 2008;24(4):493–497.
6. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N. Engl. J. Med. 2007;357(22):2277–2284.
7. Eley KA, Mcintyre AG, Watt-Smith SR, et al. “Black bone” MRI: a partial flip angle technique for radiation reduction in craniofacial imaging. Br J Radiol. 2012;85(1011):272–278.
8. Eley KA, Watt-Smith SR, Golding SJ. “Black bone” MRI: a potential alternative to CT when imaging the head and neck: report of eight clinical cases and review of the Oxford experience. Br J Radiol. 2012;85(1019):1457–1464.
9. Eley KA, Watt-Smith SR, Golding SJ. Three-Dimensional Reconstruction of the Craniofacial Skeleton With Gradient Echo Magnetic Resonance Imaging (“Black Bone”): What Is Currently Possible? J Craniofac Surg. 2017;28(2):463–467.
10. Peters DC, Korosec FR, Grist TM, et al. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43(1):91–101.
11. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341.
Figures