4702
DKI-Based Mahalanobis Distance Assessing Alterations of Brain White Matter in Infants With Benign Enlargement of Subarachnoid Space1Department of Radiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi'an, China, 2MR Research China, GE Healthcare, Xi'an, China
Synopsis
Some infants with BESS were accompanied with mildly motor and language delay. White matter (WM) development is important to neurodevelopmen, but relationships between BESS and WM maturation are not very clear. Mahalanobis distance is a feasible multivariate approach to evaluate white matter maturation. This study aims to quantitative assess the WM microstructures of in infant with BESS aged 4-6 months via DKI-based Mahalanobis distance. Larger Mahalanobis distance was found in infants with BESS among most WM tracts. All these changes of WM tracts suggested underlying alterations and prematuration of white matter. It may provide additional information for the neurodevelopment outcomes.
Introduction
Benign enlargement of the subarachnoid space (BESS) is a benign clinical entity characterized radiologically by increased cerebrospinal fluid in the subarachnoid spaces, especially overlying both frontal lobes, with a widened interhemispheric fissure and normal to slightly increased ventricular size[1]. And previous studies suggested it will be a benign self-limiting condition. But some studies found that infants with BESS may accompany with mildly motor and language delay. At present, there were few studies focused on the white matter maturation of infants with BESS. Mahalanobis distance is a feasible multivariate approach to evaluate white matter maturation by quantifying the distance between developing and matured brains [2]. Therefore, in this study, we aimed to assessing alterations of white matter in infants aged 4-6 months with BESS via diffusion kurtosis imaging (DKI) based Mahalanobis distance.Materials and Methods
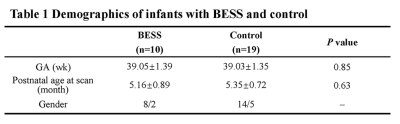
The Institutional Review Broad of the first author’s affiliation approved this study and written informed consent was obtained from parents of the children. Patients Twenty-nine infants aged 4-6 months with no abnormalities of brain on MRI were included in the study, 10 infants with BESS and 19 controls. All these infants completed scan of conventional and DKI sequences. BESS was defined as a frontal subarachnoid space or interhemispheric fissure greater than 5mm on transverse T2WI [3]. MR Protocols All subjects were examined by using a 3.0T scanner (Signa HDxt, General Electric Medical System, Milwaukee, WI, USA) with an 8-channel head coil. Data acquisition included three-dimensional fast spoiled gradient-echo T1-weighted sequence (TR/TE, 10.2ms/4.6ms; NEX, 1; isotropic 1×1×1mm3; FOV, 24cm) and transverse fast spin-echo T2-weighted sequence (TR/TE, 4200ms/116ms; NEX of 1.5; matrix, 320×320; thickness, 4mm; FOV, 24cm), followed by a DKI (25 directions; b value, 500, 1000, 1500, 2000, 2500 s/mm2; SENSE factor, 2; TR/TE, 11000/93.9ms, slice thickness, 4mm with 4mm gap, FOV, 24cm; matrix, 172×172). Data and statistical analysis DKI raw data were preprocessed by FMRIB software library (FSL; http://www.fmrib.ox.ac.uk/fsl) and 7 parameters of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) and axial kurtosisn (AK) radial kurtosis (RK), mean kurtosis (MK) were calculated by using the FMRIB Diffusion Toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT) and extract the value by MATLAB. Mahalanobis distance was derived by those 7 metrics. White matter tracts labels (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) corresponding to the FA template were successively warped to the target spaces for adults, and infants by using the deformation parameters of the FA template. The white matter structures of interest included left and right regions of 2 projection tracts: corticospinal tract at the internal capsule level (CST(IC)) and anterior thalamic radiation (ATR); 2 commissural tracts: genu of corpus callosum (GCC) and splenium of corpus callosum (SCC); left and right regions of 4 association tracts, inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), and uncinate fasciculus (UF). And cingulum (cingulate gyrus [CGC] and hippocampus[CGH]).Wilcoxon signed-rank tests were used for the differences in demographics and Mahalanobis distance between two groups. p<0.05 was considered as statistically significant difference.Results
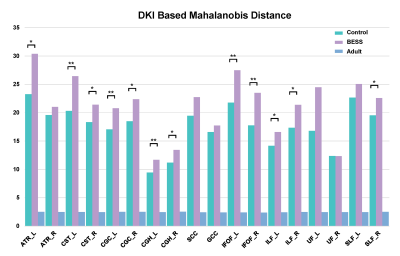
A total of 10 infants with BESS and 19 controls at age of 4-6 months were enrolled. No significant differences in GA, postnatal age at MRI scan were found between groups (Table 1). Compared with controls, Larger Mahalanobis distance was observed in infants with BESS group among left ATR, CST, CGC, CGH, IFOF, ILF, right SLF (Figure 1). The distribution of significance results were mainly in the anterior regions of brain, such as genu of corpus callosum, internal capsule and anterior corona radiata.Discussion
Enlargement of the subarachnoid space is a specific radiographic pattern, yet its pathogenesis has not been established. It may be related to arachnoid villi maturity or different growth rates of brain and skull in infants[4]. Previous study suggested FA was increased in BESS due to the compression of brain [5]. Our results also suggested the BESS has a relationship with alterations of WM, but the changes of diffusion metrics were contrary to their study. Larger Mahalanobis distance was observed in most white matter tracts, especially in CST, ILF, IFOF and cingulum. Those regions were related to the motor and cognition development. Our results suggested that the brain of infants with BESS was prematuration comparing with controls. It also may be due to the delayed brain growth and maturation resulting in the enlarged subarachnoid space. Further follow-up was needed to validate the relationships of neurodevelopment outcomes and alterations of white matter.Conclusions
White matter development was delayed in infants with BESS at age of 4-6 months. BESS may affect the white matter development. It may provide additional information for the neurodevelopment outcomes.Acknowledgements
This study was supported by the National Natural Science Foundation of China (81901516, 81971581, 81901823, 81771810 and 51706178), Shaanxi Provincial Innovation Team (2019TD-018), National Key Research and Development Program of China (2016YFC0100300), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438), the Project Funded by China Postdoctoral Science Foundation (No. 2019M653659), and the Natural Science Basic Research Plan in Shaanxi Province of China (No.2019JQ-198).References
1. Zahl SM, Egge A, Helseth E, Wester K (2011) Benign external hydrocephalus: a review, with emphasis on management. Neurosurg Rev 34 (4):417-432. doi:10.1007/s10143-011-0327-4
2. Kulikova S, Hertzpannier L, Dehaenelambertz G, Buzmakov A, Poupon C, Dubois J. Multi-parametric evaluation of the white matter maturation. Brain Struct Funct 2015;220(6):3657-72.
3. Nickel RE, Gallenstein JS (1987) Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Developmental medicine and child neurology 29 (2):181-186
4. Paciorkowski AR, Greenstein RM (2007) When is enlargement of the subarachnoid spaces not benign? A genetic perspective. Pediatr Neurol 37 (1):1-7. doi:10.1016/j.pediatrneurol.2007.04.001
5. Sun M, Yuan W, Hertzler DA, Cancelliere A, Altaye M, Mangano FT (2012) Diffusion tensor imaging findings in young children with benign external hydrocephalus differ from the normal population. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 28 (2):199-208. doi:10.1007/s00381-011-1651-2