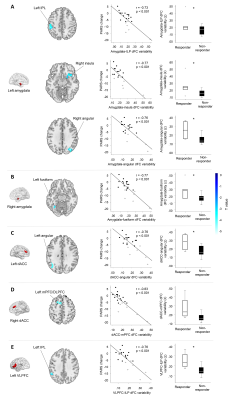
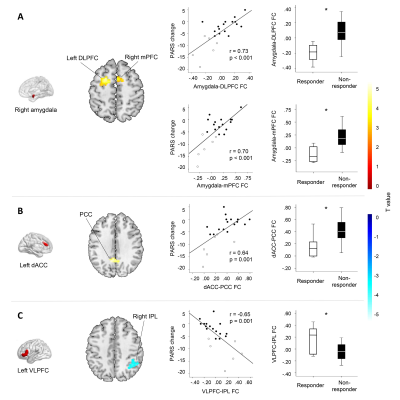
4700
The Neurobiology of Placebo Response in Adolescents with Generalized Anxiety Disorder1Huaxi MR Research Center (HMRRC), Functional and molecular imaging Key Laboratory of Sichuan Province,Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2Department of Psychiatry and Behavior Neuroscience, College of Medicine, University of Cincinnati, Cincinnati, OH, United States, 3Carl H. Lindner College of Business, University of Cincinnati, Cincinnati, OH, United States, 4Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 5Division of Child and Adolescent Psychiatry, Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York, NY, United States
Synopsis
Increasing placebo response rates represent a significant barrier to detecting treatment effects in pediatric psychiatry clinical trials. To identify biomarkers for it in adolescents with generalized anxiety disorder prior to their entering a clinical trial, whole brain dynamic and static functional connectivity (FC) were used. Dynamic and static FC between the amygdala, dorsal anterior cingulate cortex, ventrolateral prefrontal cortex and regions that subserve emotion control and inhibition were significantly associated with degree of placebo response and differed between placebo responders and non-responders. These findings may be used to decrease placebo response in clinical trials to more effectively evaluate novel treatments.
Introduction
Placebo response—symptomatic improvement patients experience while receiving placebo during clinical trials—has recently been increasing particularly in drug trials for pediatric patients with anxiety and depressive disorders.1 This increase in placebo response degrades the ability to detect treatment effects. Biomarkers that can identify placebo response in advance of treatment could markedly improve efficiency of clinical trials, enhance understanding of placebo response mechanisms, and potentially identify pediatric patients who may not need drug therapy. In the present study, we sought to identify biomarkers of placebo response in pediatric generalized anxiety disorder (GAD) using static and dynamic functional connectivity (FC) metrics as well as demographic and clinical features.Methods
Twenty-five adolescents with GAD were randomized to an 8-week placebo treatment after undergoing resting-state functional and T1 structural MR imaging on a 3 T scanner with a 32-channel phased-array head coil. Three patients were excluded because of incomplete MR data, resulting in an imaging cohort of 22 patients. Demographic and clinical characteristics of the patients are shown in Table 1. Change in Pediatric Anxiety Rating Scale (PARS) score from baseline to endpoint was used to measure placebo response. Patients with greater than 35% reduction in PARS score were defined as placebo responders. Placebo responders and non-responders did not differ in age, sex, race, full-scale IQ, baseline PARS or diagnosis, nor in terms of prior treatment (Table 1).MR data were preprocessed with the SPM12 package and DPABI toolbox using standard methods. Three regions of interest (ROI) from the amygdala-prefrontal fear circuit (amygdala, dorsal anterior cingulate cortex [dACC] and ventrolateral prefrontal cortex [VLPC]) were identified using the Brainnetome atlas.2 To detect dynamic FC variability, the sliding window approach in DynamicBC toolbox was used. The sliding window length of 20 TR (40 sec) and a step of 1 TR (2 sec) produced 121 temporal windows. In each sliding window, the temporal correlation coefficients between the truncated time course of the seeds and all the other voxels were calculated. Thus, 121 correlation maps were created for each participant. Then, a Fisher’s r-to-z transformation was applied to all the correlation maps to improve the normality of the correlation distribution. The standard deviation of the z values at each voxel was used to generate dynamic FC maps. Seed‐based static FC maps were generated for each ROI using the REST package.
Whole brain voxel‐wise multiple regression analyses were performed to examine static and dynamic FC maps in relation to placebo response. The AlphaSim approach was employed to correct for multiple comparisons, with a threshold of p < 0.005 at the voxel level and p < 0.05 at the cluster level (minimum cluster size: 78 voxels). Multiple linear regression models were used to examine the relationship between demographic and clinical variables and extent of placebo response. Finally, placebo responders and non-responders were compared (2-sample t tests) with regard to static and dynamic FC parameters that were significantly associated with placebo response. p-values <0.05 were considered statistically significant.
Results
Placebo response and baseline dynamic FCWhole-brain correlation analysis showed that greater placebo response was associated with more variable dynamic FC between left amygdala and left inferior parietal lobule (IPL), left amygdala and right insula, left amygdala and right angular gyrus, right amygdala and left fusiform gyrus, left dACC and left angular gyrus, right dACC and left medial prefrontal cortex (mPFC), and left VLPFC and left IPL. Compared with non-responders, placebo responders had significantly increased dynamic FC variability between the regions above (Figure 1, Table 2).
Placebo response and baseline static FC
Greater placebo response was associated with decreased static FC between the right amygdala and left dorsolateral prefrontal cortex (DLPFC), right amygdala and right mPFC, left dACC and bilateral posterior cingulate cortex (PCC). Placebo response was also associated with increased static FC between the left VLPFC and right IPL. Compared with non-responders, placebo responders had significantly altered static FC between those regions (Figure 2, Table 2).
Placebo response and demographic and clinical features
Placebo response was not associated with baseline age, sex, race, severity of anxiety symptom, treatment expectation, or comorbidities including presence of ADHD (Table 3).
Conclusion
Anxiety disorder is a relatively prevalent disorder that affect nearly 20% children,3 and treatment developments are needed to improve pharmacological options for this disorder. Understanding the neurobiology of placebo response is thus important. The current study is the first to examine baseline dynamic and static FC for predicting placebo response in pediatric psychiatry. Our study showed that increased placebo response was significantly associated with increased dynamic FC and decreased static FC between the amygdala, dACC, VLPFC and their functional related regions that subserve emotion control and inhibition. Moreover, there were two different brain activity patterns between placebo responders and non-responders. Specifically, increased flexibility (i.e., increased dynamic FC and decreased static FC) in placebo responders suggests that these patients may be more responsive to moment-to-moment shifts between external and internal stimuli. These findings raise the possibility that FC could serve as a useful biomarker for identifying likely placebo response, and thus be used to decrease placebo response in clinical trials to more effectively and efficiently evaluate novel treatments.Acknowledgements
Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS/NICHD) as well as Allergan, Neuronetics and Otsuka. He has received material support from and provided consultation to Myriad Genetics and receives royalties from the publication of two texts (Springer) and serves as an author for UpToDate and an Associate Editor for Current Psychiatry. Dr. Strawn also receive research support from the Yung Family Foundation. Dr. Lu has receive support from the Chinese Government Scholarship. Dr. Huang has received research support from National Nature Science Foundation (Grant NO. 81671669), Science and Technology Project of Sichuan Province (Grant NO. 2017JQ0001).References
1. Walkup JT. Antidepressant efficacy for depression in children and adolescents: Industry- and NIMH-funded studies. Am J Psychiatry. 2017. doi:10.1176/appi.ajp.2017.16091059.
2. Fan L, Li H, Zhuo J, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016. doi:10.1093/cercor/bhw157.
3. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the national comorbidity survey replication-adolescent supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010. doi:10.1016/j.jaac.2010.05.017.
Figures