4694
Confirming outcomes of disrupted white matter integrity in an independent cohort of children treated for medulloblastoma1Diagnostic Imaging, St. Jude Children's Research Hospital, Memphis, TN, United States, 2Biostatistics, St. Jude Children's Research Hospital, Memphis, TN, United States, 3Oncology, St. Jude Children's Research Hospital, Memphis, TN, United States
Synopsis
This study confirmed changes seen in a cohort of 146 patients and 92 normal healthy age-similar controls in an independent cohort of 141 patients. Fractional anisotropy (FA) was analyzed using the TBSS tools in FSL. After surgery but before any additional therapy, FA was significantly reduced in both cohorts. Additionally, a large increase in the percentage of skeleton voxels where the patients at least reach the level of controls two years from diagnosis was observed. This suggest the possibility that the newer therapy is having a less toxic impact on the microstructural integrity in this pediatric medulloblastoma population.
PURPOSE
Medulloblastoma is the most common brain tumor in children, and five-year survival has increased to over 80% in standard risk subjects.1 Effective therapy to reach this increased survival is associated with both imaging changes2 and neurocognitive deficits.3,4 In this study, we attempt to confirm earlier findings of disrupted microstructural white matter integrity in an independent cohort of children with medulloblastoma compared to age similar controls.PATIENTS AND METHODS
Three subject cohorts were utilized in this study. The historical comparison cohort (HCC) included 146 patients diagnosed with medulloblastoma (3.2-21.6 years at diagnosis; median=8.7 years; average/high-risk 93/53) and treated with maximal surgical resection, risk-adapted craniospinal irradiation (CSI), and high-dose chemotherapy (NCT00085202). Seven MRI examinations were collected: baseline (after surgery but before additional therapy); after CSI; 12, 18, 24, 30, and 36 months after diagnosis. The current treatment cohort (CTC) (NCT01878617) included 141 patients treated for medulloblastoma (3.3-22.8 years at diagnosis; median=9.3 years; average/high-risk 57/84) with surgery, CSI, and chemotherapy also. Five MRI examinations were collected for this cohort: baseline; after CSI; 12, 18, and 24 months after diagnosis. An age-similar healthy control cohort included 92 subjects (6.2-25.8 years at baseline) who were able to tolerate repeat MR scans at three time points: baseline, 12, and 24 months later. Imaging and treatment protocols were approved by the local Institutional Review Board, and written informed consent was obtained from the patient, subject, parent, or guardian, as appropriate.Diffusion tensor imaging (DTI) was collected on all subjects using a 1.5T or 3.0T whole-body system (Siemens Medical Systems, Iselin, NJ). Depending on the time of image collection, a 12 direction, four average acquisition [TR=6500-10000ms; TE=100-120ms, b=700-1000s/mm2, 1.5x1.5x3mm or 1.8x1.8x3mm resolution], a 30 direction, two average acquisition [TR/TE=14000/120ms, b=700s/mm2, 1.8x1.8x2mm resolution], or a 64 direction, two average acquisition [TR/TE=4000/77.4ms, b=1500s/mm2, 1.8x1.8x1.8mm resolution] was collected. Twelve and 30 direction acquisitions were processed with the DTI toolkit under SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) to generate maps of fractional anisotropy (FA). Sixty-four direction data was collected with reversed phase-encode blips and was therefore processed with FSL (http://fsl.fmrib.ox.ac.uk/fsl/) to generate parametric maps.5,6
Voxelwise statistical analysis of the FA data was carried out using TBSS (Tract-Based Spatial Statistics7), part of FSL.6 TBSS projects all subjects' FA data onto a mean FA tract skeleton, before applying voxelwise cross-subject statistics. After mapping the FA onto the TBSS skeleton, linear mixed-effects modeling was performed for each skeleton voxel using the restricted maximum–likelihood estimation method to analyze the longitudinal data. Estimates were computed for baseline value, change over time, and interaction between time and subject group (patient vs. control), controlling for age effects using a baseline age term for each subject. To investigate whether the change of FA values over time differs between patients and controls, we used an F-test with Kenward Roger approximation8 to find significant pixels. After correcting for multiple testing,9 p=0.05 was considered significant.
RESULTS
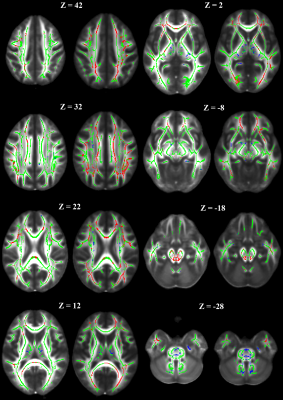
The skeleton created in the analysis of the HCC was based on 1089 examinations and included 89,456 voxels.2 The TBSS analysis of the CTC was based on 758 examinations with 99,601 skeleton voxels. Further analysis was limited to the skeleton voxels with a statistically different change in FA over time between patients and controls. While there was an 11% increase in the size of the skeleton for the CTC, the percentage of significant skeleton voxels increased from 12% in the HCC to 27% in the CTC (Table 1). These additional significant voxels can be appreciated in the display of the skeletons side by side (Figure 1). Both cohorts had greater than 95% of voxels with a baseline FA value smaller in the patient group than in controls. Breaking the significant voxels into those with a change over time greater in patients than controls, we see similar proportions of pixels from each cohort. Further examining these pixels and estimating the FA value two years after baseline shows a large increase in the percentage of pixels where the patients at least reach the level of controls.DISCUSSION
This data confirms the previously reported result that after surgery and before additional therapy, both patient cohorts have lower FA values compared to controls. This result also serves to highlight the limited impact of the various DTI acquisitions on the interpretation of results of this historical data. Additionally, we are reporting a large increase in the percentage of skeleton voxels where the patient cohort meets or exceeds the control cohort two years after diagnosis. This suggests the possibility that the newer therapy is having a less toxic impact on microstructural integrity in this pediatric population. While there are many therapy differences, the primary differences include the move from photon to proton irradiation for tumor control and a molecularly guided chemotherapy regimen.CONCLUSION
This work confirms a previous report showing a reduction of FA after surgery and before additional therapy in an independent cohort of patients treated for pediatric medulloblastoma. Additionally, preliminary analysis suggests the modifications to the molecularly guided risk adapted therapy and proton beam irradiation may result in less damage two years after diagnosis. Further study is need to confirm these findings and the impact they have on neurocognitive performance and the quality of life of these young subjects.Acknowledgements
We acknowledge the valuable contributions of Rhonda Simmons, advanced signal processing technician, and funding in part by the Cancer Center Support Grant P30 CA-21765 from the National Cancer Institute, grant HD049888 from the National Institute of Child Health and Human Development, grant RR029005 from the National Center for Research Resources, and ALSAC.References
1. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006; 7(10):813-820.
2. Glass JO, Ogg RJ, Hyun JW, et al. Disrupted development and integrity of frontal white matter in patients treated for pediatric medulloblastoma. Neuro Oncol. 2017; 19(10):1408-1418.
3. Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005; 23(24):5511-5519.
4. Brinkman TM, Krasin MJ, Liu W, et al. Long-Term Neurocognitive Functioning and Social Attainment in Adult Survivors of Pediatric CNS Tumors: Results From the St Jude Lifetime Cohort Study. J Clin Oncol. 2016; 34(12):1358-1367.
5. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003; 20(2):870-888.
6. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23 Suppl 1:S208-219.
7. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31(4):1487-1505.
8. Halekoh U, Hojsgaard S. Kenward-Roger Approximation and Parametric Bootstrap Methods for Tests in Linear Mixed Models - The R Package pbkrtest. J Stat Softw. 2014; 59(9):1-32.
9. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995; 57(1):289-300.
Figures