4692
Evaluating the Alternation of Brain Structure in Deaf Children by using Multimodal Structural Analysis1Radiology Department, The Affiliated Hospital of Yangzhou University, Yangzhou, China, 2MR Research China, GE Healthcare, Beijing, China
Synopsis
In this study, we used Surface Based Morphometry (SBM) and Tract-Based Spatial Statistics (TBSS) analysis to investigate the alternation of brain structure in deaf children. The cortical thickness of the deaf group significantly decreased in the left postcentral gyrus, superior parietal lobule, paracentral lobule, precuneus, the right transverse temporal gyri, and the middle temporal gyrus. The left precuneus local gyrification index increased. The fractional anisotropy value of the white matter in deaf children is lower than control group. These findings demonstrated that structural changes in deaf children occurred in both the grey and white matter, and revealed that the neuroplastic changes associated with cross-modal reorganization in the precuneus.
Purpose:
As reported previously, individuals with sensorineural hearing loss have thinner cortical thickness in the left precentral gyrus, the right postcentral gyrus, the left superior occipital gyrus and the left fusiform gyrus[1], and a decrease in the right cerebellar gray matter volume[2]. Diffusion Tensor Imaging (DTI) studies found significant decrease of the fractional anisotropy (FA) value in the white matter of the congenitally deaf patients, suggesting hypomyelination and/ or fewer fibers of these brain regions[3]. Given that most previous studies on brain structural changes applied the Voxel-based Morphometry (VBM) for deaf Children,, the limited explanation on brain structure and the degrees of brain change are limited. Thus, in this study, a multimodal structure analysis technology was used to systematically measure and analyze the whole brain structure of deaf children, from gray matter to white matter fibers, in order to provide a thorough brain structural basis for exploring deaf children’s cognitive and behavioral changes.Materials and Methods:
Subjects:A total of fifty-four participants were recruited for the study, including 27 deaf children aged from 9 to 13 years old and 27 gender, age-matched normal controls. The deaf group met the standardized diagnosis of hearing disability. All deaf children were intelligence-normal, right-handed, fluent in sign language and free of any other body disability, central nervous system (CNS) diseases, psychiatric disorders, or significant head trauma.
Data Acquisition:
All MRI experiments were performed on a 3T scanner (750W, GE, USA) . 3D T1-weighted anatomical images were acquired with the scan parameters of TR/TE=1900/2.52ms, TI=900ms, slice thickness=1.0mm, flip angle=9degrees, acquisition matrix=256*256. For DTI measurement, a EPI based sequence was applied with scan parameters of TR/TE=6000/80ms; direction=30, bvalue=1000s/mm, acquisition matrix=256*256.
Data preprocessing:
SBM analysis process: the data preprocessing was automatically conducted with Statistical Parametric Mapping (SPM12) software, and CAT12 toolbox package (http://dbm.neuro.uni-jena.de/cat12/) running on the Matlab platform. Diffusion Tensor Imaging data preprocessing was conducted with FSL software developed by FMRIB (Version 5.0, FMRIB, http://www.fmrib.ox.ac.uk/fsl).
Statistical analysis:
Statistical analyses for cortical thickness and gyrus index images were used surface-based linear model in CA12. Differences of FA value was calculated by the non-parametric random permutation test from the FSL software.The independent t-test was then performed to test for group differences containing age, gender and whole brain volume as covariates. The significant p threshold was set to 0.05. Threshold Free Cluster Enhancement (TFCE) method was applied to multi-correct in statistics.Results:
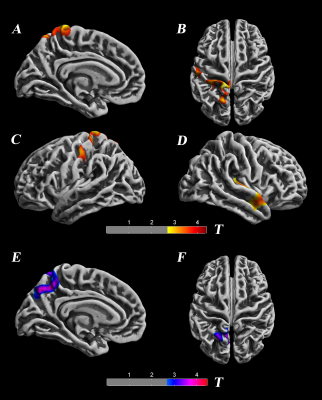
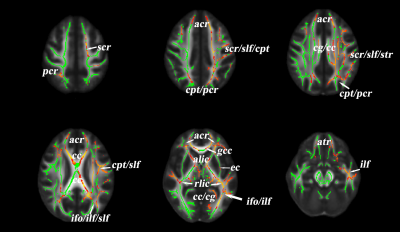
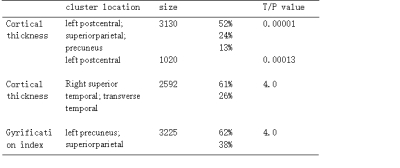
SBM analysis revealed significant decreased cortical thickness of deaf children in the left postcentral gyrus, superior parietal lobule, paracentral lobule, precuneus and the right transverse temporal gyri, middle temporal gyrus area. The local gyrification index (IGI) of the left anterior precuneus part is increased in deaf children compared to the normal controls.(p<0.05,TFCE corrected) [fig 1,table 1].Analysis of TBSS showed significant lower FA values in deaf children, including in the left anterior corona radiate, left superior corona radiate, corpus callosum, left cingulate gyrus, superior longitude fasciculus, genu of corpus callosum, anterior limb of internal capsule, retrolenticular part of internal capsule, inferior fronto-occipital fasciculus, and inferior longitudinal fasciculus..(p<0.05,TFCE corrected).[fig 2].Discussion and Conclusion:
Generally, the structure changes may be the basis for functional alternations and the functional changes also lead to the structural differences. Our findings about structural alternations in the right transverse temporal gyri, middle temporal gyrus may correlate with the loss of auditory afferent stimulation in the auditory center and the loss of hearing may lead to the structural reorganization. Our study also revealed decreased cortical thickness in the postcentral gyrus, superior parietal lobule, and paracentral lobule. Despite the decrease of cortical thickness in the left precuneus, the overall IGI increased. This shows that there are structural defects in the cortex of deaf children, and increased IGI may correlate with the need for more information integration. The loss of auditory function in deaf children may cause the brain structural remodeling and compensatory reorganization associated with vision, language and motion.Our study demonstrated different damages in the white matter, which contained many projections and contact fibers, reflecting the axonal injury and demyelination changes in a wide range of nerves. Further research should examine wether these changes resulted from the impairment of the auditory pathway, and the brain functional compensatory reorganization.To summarize, our multimodal structural analysis revealed the brain structural alternation and reorganization in deaf children, accumulating evidence for further study on correlations of hearing loss and brain structure alternation.
Acknowledgements
We confirm that the manuscript has been read and approved by all named authors and there areno known conflicts of interest associated with this publication.All protocols of this study were approved by the ethics committee of the Yangzhou University, Yangzhou, China.References
[1]. Li J, Li W, Xian J, et al. Cortical thickness analysis and optimized voxel-based morphometry in children and adolescents with prelingually profound sensorineural hearing loss[J]. Brain Res, 2012,1430:35-42. DOI: 10.1016/j.brainres.2011.09.057.
[2]. Li W, Li J, Xian J, et al. Alterations of grey matter asymmetries in adolescents with prelingual deafness: a combined VBM and cortical thickness analysis[J]. Restor Neurol Neurosci, 2013,31(1):1-17. DOI: 10.3233/RNN-2012-120269.
[3]. Liu Zh, Li M, Xian J, et al. Investigation of the white matter with tract based spatial statistics in congenitally deaf patients [J]. Chinese Journal of Medical Imaging Technology,2010,26(07):1226-1229.
Figures