4686
Optimized pediatric brain tumor diagnosis by using in vivo magnetic resonance spectroscopy and machine learning
Dadi Zhao1,2, James T. Grist1,2, Yu Sun1,2, Vijay Sawlani1,3, and Andrew C. Peet1,2
1Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 2Birmingham Children's Hospital NHS Foundation Trust, Birmingham, United Kingdom, 3Queen Elizabeth Hospital Birmingham NHS Foundation Trust, Birmingham, United Kingdom
1Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 2Birmingham Children's Hospital NHS Foundation Trust, Birmingham, United Kingdom, 3Queen Elizabeth Hospital Birmingham NHS Foundation Trust, Birmingham, United Kingdom
Synopsis
Previous studies have shown that magnetic resonance spectroscopy (MRS) can provide diagnostic classifiers for childhood brain tumors. Here we investigate the effect of noise suppression on classification and build it into a pipeline aimed at optimizing the diagnosis. Although people have proposed several algorithms to suppress noise in MRS, the clinical application is still largely restricted to apodisation. We propose a wavelet-based framework to suppress the noise of in vivo MRS, thereby the signal quality of in vivo MRS and the classification accuracy of tumor cases was improved. The framework could be used in the clinical diagnosis through MRS.
Introduction
Magnetic resonance spectroscopy (MRS) is increasingly used as a key component of pediatric brain tumor management. Accurate diagnosis of childhood cancer can be achieved by using high quality MRS1. However, the clinical use of MRS is still restricted, partially due to the presenting noise. Recently proposed methods for noise suppression in MRS were restricted to the simulation and phantom data experiments2-3. Noise suppression may aid in salvaging poor quality of in vivo MRS, and therefore improving diagnostic accuracy. Here we propose a wavelet-based framework to suppress the noise of in vivo MRS, and integrate it in a classification scheme using machine learning to optimize the accuracy of non-invasive pediatric brain tumor diagnosis for the three most common pediatric malignancies, pilocytic astrocytoma, ependymoma, and medulloblastoma.Methods
Brain tumor cases confirmed through histology were recruited and in vivo MRS was acquired using a Siemens (Munich, Germany) 1.5T scanner (PRESS, TE 30ms, TR 1500ms). Quantification was performed by using TARQUIN (version 4.3.11) with 1H brain full basis. Noise suppression was performed by using a proposed framework in the frequency domain where a series of wavelet variations was employed (Figure 1a)4. Quality control metrics used to evaluate the spectral quality with overall and maximum signal-to-noise ratios as well as full-width at half maximum (FWHM).Metabolite concentrations were normalized based on the sum of all available metabolites. Whole spectra were constructed by phased real spectra sampled from 0~4ppm, which were then normalized and baseline subtracted. Feature extraction was performed by selecting metabolites with promising area under the receiver operating curve (AUC>0.7) and acceptable fitting performance based on Cramér–Rao lower bound (SD<200%). Whole spectra were dimensionality reduced by using principal component analysis.
Mann-Whitney U test was performed to measure the improvement of quality control metrics. Multi-class receiver operating characteristics5 was performed to measure the diagnostic performance of single metabolites among the three groups of tumor. Based on the MRS data before and after noise suppression, a comparative classification was performed through linear discriminant analysis (LDA), support vector machine (SVM) and multinomial log-linear model based single-layer neural network. Oversampling for the ependymoma group was performed by using SMOTE6 and wavelet oversampling (WTOS). In wavelet oversampling, top outputs by the wavelet framework were defined as oversampled metabolite profile or spectra. The classification accuracy was evaluated by using leave-one-out cross validation. All statistics and machine learning experiments were processed in R (version 3.5.3).
Results
Eighty-three eligible cancer cases were enrolled to the study (11 ependymomas, 33 medulloblastomas, and 39 pilocytic astrocytomas). After noise suppression, in vivo MRS showed improved signal quality, with an example shown in Figure 1b. The diagnostic performance of individual metabolites (AUC>0.7) saw a small increase after noise suppression (Figure 2a). Quality control metrics were also significantly improved (P<0.05, Figure 2b). All classification methods suggest subsequent increase in classification accuracy of pediatric brain tumors after noise suppression by using both metabolite concentrations and whole spectra (Figure 3). The neural network and SVM classifier perform best in metabolite-based (87.9%) and spectra-based (86.7%) classification, respectively. Improved classification performance was achieved by over sampling the ependymoma group using either metabolite concentrations or whole spectra. Wavelet oversampling outperformed than SMOTE in the whole spectra based classification (Figure 4). The best classification accuracy achieved was 90.4% by the top six metabolites with 200% wavelet oversampling and SVM. For whole spectra the best classification accuracy was 88.0%, achieved by using five principal components with 300% wavelet oversampling and LDA.Discussion
This study has focused on using a wavelet framework to improve in vivo MRS signal quality, and thereby the diagnosis of pediatric brain tumours through machine learning. Optimal noise suppression was achieved by using adaptive wavelet variations. The quality of MRS signals was improved, as determined by signal-to-noise ratios and line width. Noise suppressed MRS provided metabolites with increased AUC, potentially indicating improved signal quality, reliability and diagnostic performance of metabolite concentrations.An increased number of metabolites meeting our AUC criteria with improved diagnostic performance after noise suppression indicated better signal quality and reliability for identifying brain tumors through metabolite profiles. Both metabolite concentration and whole spectra based classification showed improved classification accuracy in noise suppressed MRS (Figure 3). Limited ependymoma cases indicate a problem of imbalanced learning. Oversampling through SMOTE and WTOS aided in addressing the problem, as reflected by the improved classification performance (Figure 3a, Figure 4a). Future work will expand the project to multi-site 3T MRS.
Conclusion
Many elements have been involved for optimizing the diagnostic performance of in vivo MRS for non-invasive pediatric brain tumor diagnosis. The improved classification accuracy made a path to the possible use of this framework of noise suppression into clinical practice.Acknowledgements
This study was funded partially by the Help Harry Help Others doctoral studentship (D.Z.) and the Little Princess Trust (J.G.).References
- Davies NP, Wilson M, Harris LM et al. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR in Biomedicine 2008; 21: 908-918.
- Antoine JP, Chauvin C, Coron A. Wavelets and related time-frequency techniques in magnetic resonance spectroscopy. NMR in Biomedicine 2001; 14: 265-270.
- Ahmed OA. New denoising scheme for magnetic resonance spectroscopy signals. IEEE Transactions on Medical Imaging 2005; 24: 809-816.
- Zhao D, Grist JT, Sun Y et al. Improved classification of paediatric brain tumours through whole spectra from in vivo magnetic resonance spectroscopy. Proc Intl Soc Mag Reson Med 2019; 27: 3083.
- Hand DJ, Till RJ. A simple generalisation of the area under the ROC curve for multiple class classification problems. Machine Learning 2001; 45: 171-186.
- Chawla NV, Bowyer KW, Hall LO et al. SMOTE: synthetic minority over-sampling technique. Journal of Artificial Intelligence Research 2002; 16: 321-357.
Figures
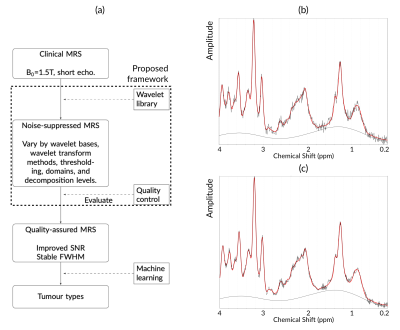
The pipeline of the project (a), as well as an example of noisy (b) and noise suppressed MRS (c).
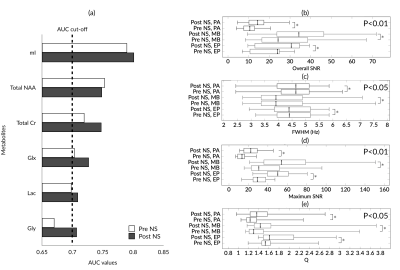
Metabolite concentration statistics by multi-class receiver operating characteristics (a), and improvement of quality control metrics of clinical MRS by using proposed method (b-e). Abbreviations: mI, myo-Inositol; NAA, N-acetylaspartate; Cr, creatine; Glx, glutamate and glutamine; Lac, lactate; Gly, glycine; EP, ependymoma; MB, medulloblastoma; PA, pilocytic astrocytoma; SNR, signal-to-noise ratios; AUC: area under the curve.
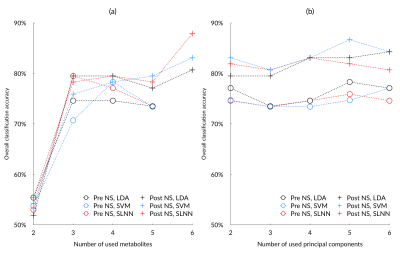
Performance of metabolite-based (a) and spectra-based (b) tumour classification based on pre and post noise suppression (NS) MRS, using linear discriminant analysis (LDA), support vector machine (SVM), and single-layer neural network (SLNN).
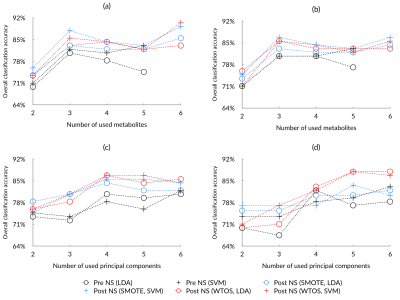
Performance of oversampling based on SMOTE or wavelet oversample (WTOS) by metabolite-based (a, b) and spectra-based (c, d) tumour classification with linear discriminant analysis (LDA) and support vector machine (SVM), comparing 200% (a, c) and 300% (b, d) oversampling for ependymomas as well as pre and post noise suppression (NS) MRS.