4685
Uptake of MRI and associated data quality in an inclusive clinical trial in children with Autism Spectrum Disorder1University of Sydney, Camperdown, Australia, 2Brain and Mind Centre, University of Sydney, Camperdown, Australia
Synopsis
Individuals with Autism Spectrum Disorder (ASD) show high rates of comorbidities, including intellectual disability. Often, individuals with comorbid conditions are excluded from clinical trials and neuroimaging studies, potentially biasing the development of treatments and subtypes. In an inclusive trial (without functioning constraints) for children with ASD (aged 3-12 years), 71 participants consented to MRI and 24 (34%) completed an MRI scan, following a familiarization procedure. Twenty-one participants had a successful post-treatment MRI. This study reports on the resultant data quality and shows the potential to include MRI in trials of complex populations who, typically, are excluded.
Introduction
Autism Spectrum Disorder (ASD) is a diagnosis based on social communication difficulties as well as restricted and repetitive behavior and interests [1]. At present, there is no pharmacological treatment for the core social symptoms of ASD [2]. A key issue in ASD research is the identification of unbiased measures to predict treatment response and evaluate intervention outcomes [3].MRI is a technique proposed to overcome these limitations by providing direct insight into the effects of intervention on the brain. ASD research is increasingly using MRI, including to predict and measure treatment response [4]. Studies typically restrict enrolment to those with specific adaptive functioning or intelligence scores, which results in studies of high-functioning ASD groups, and not representing the full spectrum of the disorder [4]. This is understandable given the need for participant compliance in order to acquire good quality data and the cost of acquiring MRI data. However, this approach severely limits the generalizability of findings as these restrictions exclude a significant proportion of individuals with ASD, for example the 30-55% with comorbid intellectual disability [5, 6]. This likely does not reflect biological subtypes. For instance, a recent study evidenced distinct patterns of functional activity in individuals with ASD with comorbid intellectual disability when compared to those with normal intelligence [7].
With the motivation to better describe the diverse presentations of ASD, this study reports on the feasibility of MRI scanning in a comprehensive, inclusive clinical trial investigating oxytocin for children (aged 3-12 years) with ASD. Of note, no functional or intelligence capacity inclusion/exclusion criteria were applied nor were sedatives used.
Methods
This MRI study is a sub-study of a larger ASD clinical trial investigating intranasal oxytocin (ACTRN12617000441314). For the main trial, participants aged 3-12 years who met DSM-5 criteria for ASD were recruited. The only exclusion criteria were medical and related specifically to the nasal spray administration.MRI Procedures
At the first two visits, participants were offered the opportunity to participate in a mock, or practice, scanning procedure. The only additional exclusion criteria beyond the main trial were for MRI safety. These mock scans were designed to emulate the process of having a real MRI as much as possible. Following the mock scan, a decision as to whether to progress to the real scan was made. The real scan was completed pre- and post- treatment. The trial outline is detailed in Figure 1 and the details of participants, divided by MRI participation, are outlined in Figure 2.
The MRI protocol consisted of localizer and ASSET calibration scans, a T1-weighted FSPGR anatomical (4min 24sec), a PRESS acquisition placed in the anterior cingulate cortex (ACC) (5min 4sec), a MEGA-PRESS acquisition in the parietal lobe (8min 24sec) GABA quantification and, a DWI-scan (10min 44 sec). In cases of obvious motion on the T1-weighted scan, a repeated MRI attempt was performed up to 3 times.
Quality checks
Anatomical: The best quality anatomical image was selected and then passed to an automated MRI quality control process [8]. Output from the group quality control check is included in Figure 4.
ACC PRESS: The ACC PRESS was processed using LCModel [9]. Quality was assessed using a SNR value >15 and a FWHM < 0.1 [10] as well as visual inspection.
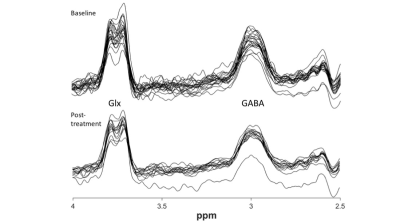
GABA MEGA-PRESS: MEGA-PRESS data were processed using Gannet [11], version 3.1.3. Metrics for quality were developed in line with the Big GABA [12] paper, along with visual inspection. Data is presented in Figure 5.
DWI: The raw images of the diffusion data were visually inspected and, as per [13], datasets with ≥5 corrupted volumes were discarded. Datasets were double-checked by generating fractional anisotropy (FA) maps using MRTRIX3 [14].
Results
Seventy-one participants consented to the MRI component of the protocol. Of these, 8 did not engage with the familiarization routine, 39 attempted familiarization but did not progress to the real MRI and 24 successfully completed an MRI scan at baseline. Post-treatment, 22 participants returned, with 21 successful scan completions. The data quality for each MRI acquisition is detailed in Figure 3.Discussion
This study examined the feasibility of including MRI scanning in a comprehensive, inclusive clinical trial for children with ASD, and reports on the data quality for those who completed scanning. This is the first study (to our knowledge) to report on neuroimaging acquisition in an inclusive clinical trial in ASD without any intelligence or adaptive functioning score restrictions. The acceptability of participating in the scan portion of our study is highlighted by our high levels of retention. The two participants that were lost to follow-up discontinued from the trial as a whole, not just the MRI component.Of those participants who were able to complete scanning at the initial MRI, almost all were able to participate in the post-treatment MRI. Although no participant was refused entry into the trial due to inability to complete any of the behavioral or biological measures, the MRI data successfully collected was obtained from those participants with higher intelligence and lower ASD severity scores. This study highlights the potential for more representative trials in ASD, that include measures such as MRI, even if not all participants complete every component.
Acknowledgements
We acknowledge a BUPA Foundation Grant to Adam J. Guastella to investigate markers and methods of oxytocin and an Endeavour Foundation Grant to develop the procedures described. We also acknowledge Project Grants (1043664 and 1125449) to Adam J. Guastella, a NHMRC senior principal research fellowship (APP1136259) to Ian B. Hickie and a Research Training Program Fellowship to Marilena M. DeMayo (SC0042/SC1999). We wish to thank both Kevin Pelphrey and his staff and former colleagues at Brown University for training provided to MDM. Finally, we wish to acknowledge the assistance of the staff at i-Med Radiology, Camperdown, for assistance with data collection.
We thank Mark Mikkelsen and Ashley Harris for their comments.
References
1. APA, DSM 5. 2013. 991-991. 2. Masi, A., et al., An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci Bull, 2017. 33(2): p. 183-193. 3. DeMayo, M.M., et al., Circuits for social learning: A unified model and application to Autism Spectrum Disorder. Neurosci Biobehav Rev, 2019. 107: p. 388-398. 4. Calderoni, S., et al., Rehabilitative Interventions and Brain Plasticity in Autism Spectrum Disorders: Focus on MRI-Based Studies. Frontiers in Neuroscience, 2016. 10(139). 5. Charman, T., et al., IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol Med, 2011. 41(3): p. 619-27. 6. Loomes, R., L. Hull, and W.P.L. Mandy, What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 2017. 56(6): p. 466-474. 7. Gabrielsen, T.P., et al., Functional MRI connectivity of children with autism and low verbal and cognitive performance. Mol Autism, 2018. 9: p. 67. 8. Esteban, O., et al., MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One, 2017. 12(9): p. e0184661. 9. Provencher, S.W., Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine, 2001. 14(4): p. 260-264. 10. Yang, Z.Y., et al., A quantitative analysis of (1)H-MR spectroscopy at 3.0 T of three brain regions from childhood to middle age. Br J Radiol, 2015. 88(1052): p. 20140693. 11. Edden, R.A., et al., Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging, 2014. 40(6): p. 1445-52. 12. Mikkelsen, M., et al., Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage, 2017. 159: p. 32-45. 13. Nordahl, C.W., et al., Methods for acquiring MRI data in children with autism spectrum disorder and intellectual impairment without the use of sedation. J Neurodev Disord, 2016. 8(1): p. 20. 14. Tournier, J.D., F. Calamante, and A. Connelly, MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 2012. 22(1): p. 53-66.Figures
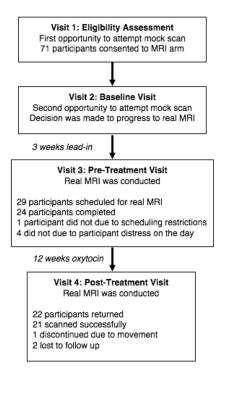
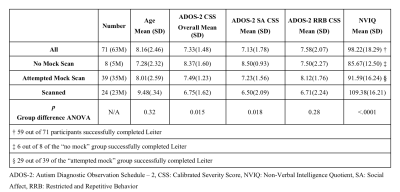
Details of participants who consented to the trial, those who completed no mock scans, attempted mock scans and completed real scans.
Abbreviations: ADOS-2: Autism Diagnostic Observation Schedule – 2, ANOVA: Analysis of Variance, CSS: Calibrated Severity Score, NVIQ: Non-Verbal Intelligence Quotient, SA: Social Affect, RRB: Restricted and Repetitive Behavior.
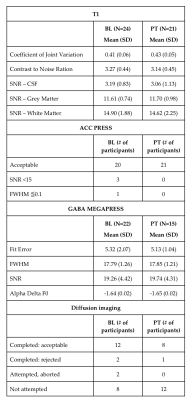
Data acquired and associated quality from each acquisition type.
Abbreviations: BL: baseline, CSF: cerebrospinal fluid, FWHM: full-width half-maximum, PT: post-treatment, SNR: signal-to-noise ratio
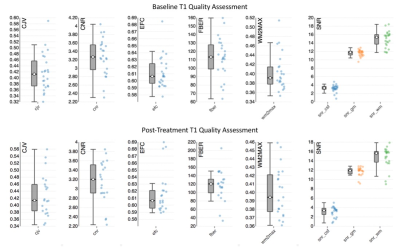
Interquartile range and range of quality assessment metrics from the automated pipeline. Tails are minimum and maximum and the boxplot represents interquartile range and median.
Abbreviations: CJV: coefficient of joint variation, CNR: contrast-to-noise ratio, CSF: cerebrospinal fluid, EFC: entropy focus criterion, FBER: foreground-to-background energy ratio, GM: grey matter, SNR: signal-to-noise ratio, WM: white matter, WM2MAX: white-matter to maximum intensity ratio.