4682
Characterization of Gray and White Matter Myelination Across Adolescence Using Macromolecular Proton Fraction Mapping1Institute for Learning and Brain Sciences, University of Washington, Seattle, WA, United States, 2Radiology, University of Washington, Seattle, WA, United States
Synopsis
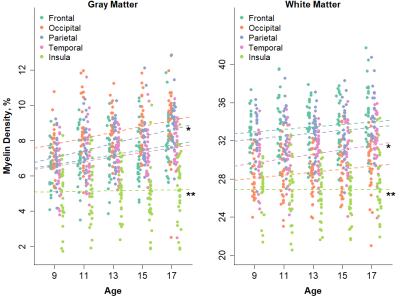
Although previous studies have investigated brain structural changes across adolescent development, little attention has been paid to the myelination that occurs in the gray matter during this period. We utilized macromolecular proton fraction mapping to investigate myelination of gray and white matter in a cross-sectional sample of 146 adolescents at 9, 11, 13, 15 and 17 years of age. Throughout most of the brain, gray matter myelin density was found to increase at a faster rate with age than white matter myelin density. Our findings suggest that gray matter myelination is a significant component of brain maturation during adolescent development.
Introduction
Adolescence is a period of marked psychological, cognitive and social transition in which there are changes to both the gray and white matter of the brain. Previous research on the adolescent brain has largely been focused on characterizing myelination of white matter as well as changes in cortical gray matter thickness and volume. Little attention has been paid to the myelination that occurs in the gray matter during this developmental period. Macromolecular proton fraction (MPF) mapping provides a means to quantitatively measure myelination of both the gray and white matter in vivo at high resolution throughout the brain. MPF values are directly related to myelin content, and are insensitive to iron deposition in the brain.1-3,5 The aim of this study was to use MPF mapping to investigate how myelin density in both the gray and white matter changes across adolescence, and to compare relative rates of myelination of gray and white matter in different brain regions during adolescent development.Methods
MPF data were collected on a 3T Philips Ingenia scanner from a cross-sectional sample of 146 adolescents at 9, 11, 13, 15 and 17 years of age. Acquisition and reconstruction of MPF maps with a resolution of 1.25 mm3 was carried out according to the single-point synthetic reference method using a protocol previously detailed.4 Average myelin density within gray and white matter was estimated for each individual in all lobes of the brain, as well as in the caudate, putamen and thalamus, using a linear recalculation formula.1 Our primary analysis examined the relationship between average regional myelin density and age using a general linear model, with gender included as a covariate. The Bonferroni correction was used to account for multiple testing in the primary analysis. A complementary voxel-level analysis was then conducted to generate statistical maps of correlations of MPF with age using the RANDOMISE toolbox of the FSL software package. These maps were generated using threshold free cluster enhancement and a significance threshold of 0.05. In addition, we explored the relationship between gray matter myelin density and reading comprehension skill using a general linear model, with age as a covariate.Results
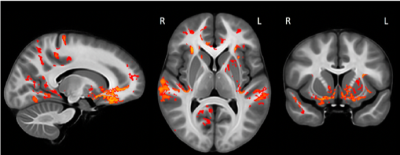
Increases in myelination were observed in both the gray (p=0.002) and white matter (p=0.050) of the brain. The rate of myelination was found to vary according to anatomical region and compartment (Figure 1). Gray matter myelin density was found to increase with age at a faster rate than white matter myelin density (rate: 1.7 vs. 0.4% per year, p=0.002). Within the gray matter, myelin density in the parietal lobe was found to increase at a significantly faster rate than myelin density in all other lobes (rate: 2.5 vs. 1.5% per year, p<0.001). Within the white matter, myelin density in the temporal lobe was found to increase at a significantly faster rate than myelin density in all other lobes (rate: 0.8 vs. 0.5% per year, p<0.001). Myelin density of the insular gray (rate: 0.2 vs. 2.1% per year, p=0.009) and white (rate: 0.0 vs 0.5, p=0.003) matter was found to proceed at a significantly slower rate than all of the other lobes. The voxel-level analysis identified specific regions within each lobe where myelination was most strongly correlated with age (Figure 2). These included the orbitofrontal cortex, the frontal pole, the precuneus, the somatosensory cortex, the superior temporal gyrus, and the putamen. As part of an exploratory analysis, the partial correlation between gray matter myelin density in the superior temporal gyrus and scores on a reading comprehension task, controlling for age, was found to be significant (p=0.048).Discussion
Synaptic pruning, cortical myelination, and microstructural proliferation have all been proposed as mechanisms that underlie observed decreases in volume and thickness of the cerebral cortex during the transition from childhood and adulthood.6,7 Utilizing quantitative T1 and T2 imaging, other groups have recenty investigated the mechanisms that are involved in the restructuring of the cortex across this time period.6,7,8 However, these techniques are limited by their inability to directly measure myelin content, as well as their sensitivity to iron, which is distributed throughout the cortex and subcortical regions. MPF mapping is insensitive to iron5 and is highly reflective of myelin content. We found the rate of gray matter myelination to exceed that of white matter myelination in general throughout the brain. Our findings indicate that gray matter myelination is a significant part of the restructuring of the cortex during adolescent development. Increased myelination of the gray matter may reflect increased short-distance intracortical connections, and may also have significance in terms of synaptic plasticity.9 Our finding of a correlation between gray matter myelination and behavior suggests functional significance to the gray matter myelin changes that are consistent with previous reports8 and merits further exploration.Conclusion
These findings support the conclusion that myelination of gray matter is a significant component of brain maturation during adolescent development. They also add to a small but growing body of literature that suggests that restructuring of the gray matter during adolescent development may be more complex than previously recognized. Further investigations into the changes in gray matter myelination during adolescence may yield valuable insight into its functional significance.Acknowledgements
We would like to thank Dr. Baocheng Chu and Dakota Ortega for valuable assistance with data collection.References
1. Underhill HR, Rostomily RC, Mikheev AM, et al. Fast bound pool fraction imaging of the in vivo rat brain: Association with myelin content and validation in the C6 glioma model. Neuroimage 2011;54(3):2052-2065.
2. Khodanovich MY, Sorokina IV, Glazacheva VY, et al. Histological validation of fast macromolecular proton fraction mapping as a quantitative myelin imaging method in the cuprizone demyelination model. Sci Rep 2017;7:46686.
3. Yarnykh VL, Bowen JD, Samsonov A, et al. Fast whole-brain three-dimensional macromolecular proton fraction mapping in multiple sclerosis. Radiology 2015;274(1):210-220.
4. Yarnykh VL. Time-efficient, high-resolution, whole brain three-dimensional macromolecular proton fraction mapping. Magn Reson Med 2016;75(5):2100-2106.
5. Yarnykh VL, Krutenkova EP, Aitmagambetova G, et al. Iron-insensitive quantitative assessment of subcortical gray matter demyelination in multiple sclerosis using the macromolecular proton fraction. AJNR Am J Neuroradiol 2018;39(4):618-625.
6. Gomez J, Barnett MA, Natu V, et al. Microstructural proliferation in human cortex is coupled with the development of face processing skills. Science 2017;355(6320):68-71.
7. Natu VS, Gomez J, Barnett M, et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Science 2019;116(41):20750-20759.
8. Grydeland H, Walhovd KB, Tamnes CK, et al. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2- weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci 2013;33(47):18618-18630.
9. Glasser MF, Goytal MS, Preuss TM, et al. Trends and properties of human cerebral cortex: correlations with cortical myelin content. Neuroimage 2014;93(2):165-175.
Figures