4681
Characterization of brain metabolite changes in early childhood1Radiology, University of Calgary, Calgary, AB, Canada, 2Alberta Children's Hospital Research Institute and the Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
Synopsis
Using proton MRS, we examine longitudinal changes in tNAA, tCr, tCho, Ins and Glx in early childhood in a typically developing children ages 2.4-9.3 years. We show NAA increases in the anterior cingulate across both sexes while age-relate changes in NAA in the left angular gyrus are only seen in boys. In the anterior cingulate tCr in girls increases and tCho decreases in boys. By contrast in the left angular gyrus tCr increases in boys and tCho decreases in girls. These different metabolite trajectories in different brain regions may be associated with different development seen between boys and girls.
Introduction
The brain undergoes rapid changes in early childhood, including changes in cortical thickness [1], white matter microstructure [2], and functional connectivity [3]. Magnetic resonance spectroscopy (MRS) provides information on brain neurochemistry, which can provide new insight to the mechanisms underlying brain disorders and disease as well as potential targets for therapy. Previous studies have shown dramatic metabolite changes in the first 3 months of life, followed by increasing N-Acetyl Aspartate (NAA) and relatively stable trajectories of other metabolites in childhood [4]. Studies have also shown lower NAA and glutamate and higher choline in infants compared to children [5], and increasing NAA, glutamate/glutamine (Glx) and total creatine (tCr) in late childhood [6]. These studies show ongoing changes in childhood, but have had few participants in the early childhood range, making it difficult to detect any age-related changes that occur; furthermore, sex differences have not been examined. Characterizing typical changes in metabolites will assist with understanding this important developmental phase as well as identifying deviations from normal trajectories that may occur in brain disorders, disease, or injury.Methods
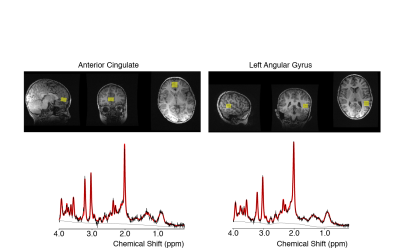
This study includes 341 scans from 119 children (2.4-9.3; 4.3 +/- 1.3 years; 60 female/59 male). Children were recruited between 2-5 years and invited to return approximately every 6 months; not all children provided magnetic resonance spectroscopy (MRS) data at each visit. MRS data was collected using Point RESolved Spectroscopy (PRESS) (TR = 2s, TE = 35 ms, 96 averages, 2 x 2 x 1.5 cm3 voxels). At the first visit, MRS data was acquired in the anterior cingulate gyrus (Figure 1) and then was collected in the left angular gyrus (Figure 1) for most follow-up visits. The data analyzed here are 102 datasets (92 participants; 2.4-8.0 years) from the anterior cingulate gyrus and 239 datasets (81 participants; 4.1-9.3 years) from the left angular gyrus. MRS data was pre-processed including spectral registration and phasing using FID-A [7] and metabolites were quantified using LCModel [8] using the default basis set including 18 metabolites and 9 macromolecule/lipid peaks. The metabolites quantified and analyzed were: total N-acetyl aspartate (tNAA, NAA+NAAG), total creatine (tCR, creatine + phosphocreatine), total choline (tCho, glycerophosphocholine + phosphocholine), inositol (Ins) and Glx (glutamate+ glutamine). Once metabolites were quantified, linear mixed effects analysis was performed with SPSS including age, sex, and their interaction as fixed effects, and subject as a random effect, which accounts for the repeated measures. In cases where the age-sex interaction was not significant, it was removed the model was rerun. False discovery rate (FDR) was used to account for 10 multiple comparisons (5 metabolites x 2 voxel locations).Results: Anterior Cingulate (Figure 2)
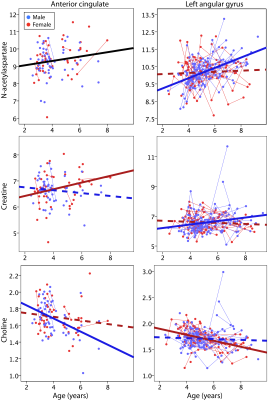
For tNAA, there was a positive main effect of age (p=0.039) that did not survive multiple comparison correction. tCR showed a significant interaction between sex and age (p=0.047), though again, this finding did not survive FDR correction. There was a significant negative main effect of age on tCho (p=0.003). There were no significant effects of age on Ins or Glx.Results: Left Angular Gyrus (Figure 2)
tNAA had a significant age-sex interaction (p<0.001), where tNAA significantly increased with age in males but remained stable in females. tCr also had a significant age-sex interaction (p=0.018) with similar age-related increases in males and no change in females. tCho had a significant age-sex interaction (p=0.01) where females showed age-related decreases, but males showed no changes.Discussion
Here we show age-related changes in tNAA (a marker of neuronal viability, density and health), tCr (a marker of bioenergetics) and tCho (a marker for membrane turnover) that vary between boys and girls, and between brain regions. tNAA increased with age in the anterior cingulate in both boys and girls, but tNAA increased in the left angular gyrus only in boys. As tNAA is a neuronal marker and is involved in metabolism this may indicate the on going development and specialization within these brain regions. tCr increased in girls in the anterior cingulate, but in boys in the left angular gyrus, indicating differential development of bioenergtic pathways in these regions across the sexes. tCho decreased in boys in the anterior cingulate, and in girls in the left angular gyrus. The NAA and tCr results agree well with previous studies showing increases during infancy or later childhood [4-6], helping to further understand the specific trajectories in early childhood. We also show age-related decreases in tCho in childhood for the first time. Interesting sex-age interactions were observed that differed by region. Boys showed bigger changes of tCho in the anterior cingulate and tNAA and tCr in the left angular gyrus, while girls showed bigger changes of tCr in the anterior cingulate and tCho in the left angular gyrus. These may be influenced by variations in development trajectories between boys and girls, and among different brain regions. In general, girls appear to have earlier brain development [9], and parietal brain regions develop before frontal areas [10]. This is the first, large study to examine the relationship between metabolite levels and preschool development. We show that males and females have different metabolite trajectories in different areas of the brain which may be associated with the differential developmental milestones seen in boys and girls.Acknowledgements
Funding for this study was provided by the Canadian Institutes of Health Research.References
1. Remer J, Croteau-Chonka E, Dean DC, 3rd, et al. Quantifying cortical development in typically developing toddlers and young children, 1-6 years of age. Neuroimage. 2017;153:246-261.
2. Reynolds JE, Grohs MN, Dewey D, Lebel C. Global and regional white matter development in early childhood. Neuroimage. 2019;196:49-58.
3. Long X, Benischek A, Dewey D, Lebel C. Age-related functional brain changes in young children. Neuroimage. 2017;155:322-330.
4. Bluml S, Wisnowski JL, Nelson MD, Jr., et al. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex. 2013;23(12):2944-2955.
5. Degnan AJ, Ceschin R, Lee V, Schmithorst VJ, Bluml S, Panigrahy A. Early metabolic development of posteromedial cortex and thalamus in humans analyzed via in vivo quantitative magnetic resonance spectroscopy. J Comp Neurol. 2014;522(16):3717-3732.
6. Holmes MJ, Robertson FC, Little F, et al. Longitudinal increases of brain metabolite levels in 5-10 year old children. PLoS One. 2017;12(7):e0180973.
7. Simpson R, Devenyi GA Jezzard P et al. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2017; 77(1):23-33.
8. Provencher SW, Estimation of Metabolite Concentrations from Localized in Vivo Proton NMR Spectra. Magn Reson Med. 1993; 30(6): 672-679.
9. Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065-1073.
10. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174-8179.
Figures