4680
Comparison of adipocyte size measurements with histology and high b-value diffusion-weighted spectroscopy in human white adipose tissue at 3 T1Department of Diagnostic and Interventional Radiology, Technical University of Munich, Munich, Germany, 2Else Kröner Fresenius Center for Nutritional Medicine, Technical University of Munich, Freising, Germany
Synopsis
Despite its high relevance in metabolic research, the non-invasive measurement of adipocyte size remains an unmet need. DW-MRS has been previously applied to probe diffusion restriction effects in-vivo to measure lipid droplets in animals up to diameters of 10 µm and in humans up to diameters of 50 µm. However, probing diffusion restriction in white adipose tissue, consisting of adipocytes up to a size of 150 µm, on a clinical system remains a major challenge. This work proposes to measure adipocyte sizes with high b-value long diffusion time DW-MRS. In a preliminary analysis, the presented method matches with histology.
Purpose
The measurement of lipid droplet size is important in the study of adipose tissue (AT) and ectopic lipids in both health and metabolic dysfunction across organs and tissues. In white AT, enlarged adipocyte size has been linked to the obese phenotype1. However, the assessment of AT droplet size currently requires a highly invasive biopsy procedure. Diffusion-weighted (DW) magnetic resonance (MR) is a powerful tool for the non-invasive assessment of tissue microstructure. The reduction of the apparent diffusion coefficient with increasing diffusion times due to diffusion restriction effects has been previously applied to extract cell size in water-containing tissues 2. Measuring the diffusion properties of lipids has proven to be challenging because fat has a diffusion coefficient approximately two orders of magnitude lower than water3,4. The low lipid diffusion coefficient increases the required diffusion weighting, inducing technical challenges related to eddy currents5 and increased sensitivity to motion6. Single-voxel DW-MR spectroscopy (DW-MRS) has been successfully applied to investigate lipid diffusion properties3 and was recently applied to probe lipid diffusion restriction effects in water-fat phantoms7and in animals to measure lipid droplets up to a diameter of 10µm8,9 and in humans up to a diameter of 50µm7. In subcutaneous white adipocytes diameters up to 150µm10 have been reported. So far, the measurement of white adipocytes with DW-MR lacks ex-vivo comparison with a gold-standard. The ex-vivo validation is especially challenging because restriction effects are small due to the large adipocytes size and the low lipid diffusion constant at room temperature. The purpose of the present work is to apply DW-MRS to measure adipocyte size in ex-vivo human tissue samples and to compare the results with histology as the gold-standard.Methods
Ex-vivo human tissue samplesSubcutaneous AT samples from the abdominal area were obtained from two patients undergoing abdominoplasty. Two approximately 10x10x5cm3 tissue specimens fixed in formaldehyde (4%) were used for MR measurements and small samples were extracted for histology.
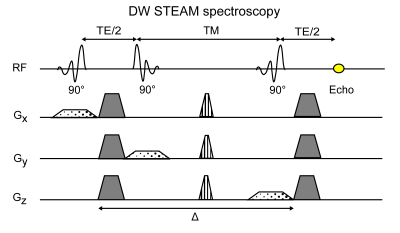
Pulse sequence and MRS post processing
The two samples were scanned on a decoupling table to mitigate vibration artifacts using an 8-channel extremity coil with a clinical 3T system (Ingenia Elition, Philips Healthcare, Best). A bipolar DW-STEAM-MRS sequence (Figure 1) with the following sequence parameters was acquired: TE/TR: 70/1800ms, b-values: 20,000/40,000/60,000/80,000s/mm2, 8 averages per b-value and diffusion gradient polarity, VOI: (20mm)3,TM: 300ms to 700ms in 25ms steps, scan time: 2:02min per diffusion time.The slew rate of the diffusion gradients was limited to 20 mT/ms/m to further reduce vibration artifacts. The pre-processing of the spectra included zero order phasing and gaussian apodization. The single averages were frequency aligned and eddy current corrected before averaging. Peak area quantification was performed considering eight fat peaks11. Only the methylene (1.3ppm) peak area was used for the adipocyte size estimation. For each diffusion time an exponential fit was performed to extract the apparent diffusion coefficient (ADC). The ADC decay for increasing diffusion times was fitted with the restricted diffusion behavior in the short diffusion time regime according to Mitra12. The standard deviation of the adipocyte estimation was calculated as 3.92x95% confidential interval of the fitting.
Validation measurements
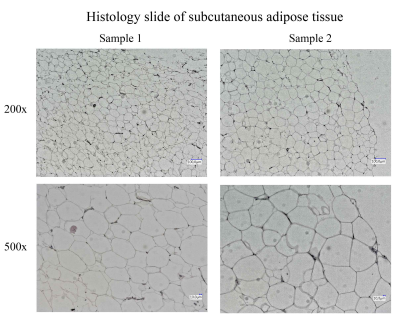
After slicing and staining with hematoxylin and eosin, images were taken in High Definition Range (HDR) at 200 x magnification (VHX-6000, Keyence, Japan). Adipocyte size determination was carried out using the proprietary microscope image analysis software. Adipocyte diameter was calculated from the obtained area. Figure 2 shows an exemplary histology slide for each sample.
Results
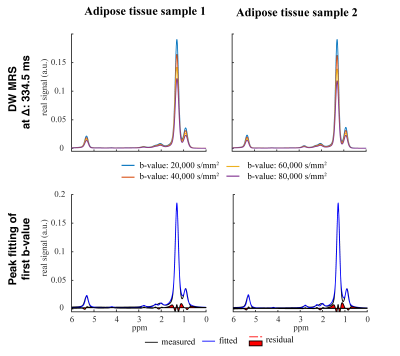
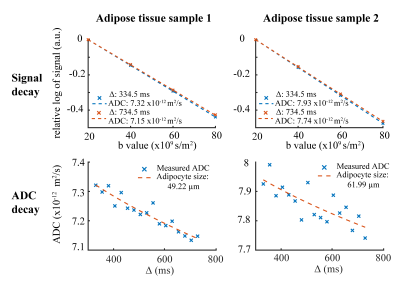
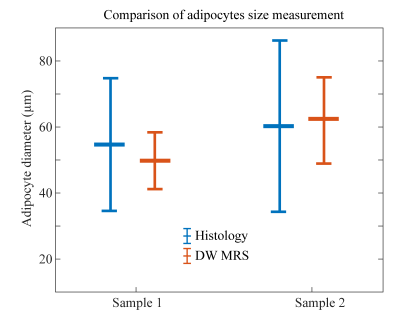
In Figure 3, exemplary DW spectra and the fitting are shown. Figure 4 shows the decay of the methylene peak area as a function of the b-value at the longest and shortest diffusion time and the ADC decay with corresponding fitting as a function of the diffusion time. In sample 1 the MRS-based mean diameter was estimated to be 49.2±4.4µm and in sample 2 to be 62.0±13.1µm. Figure 5 shows the agreement in the adipocyte size measurement between the histology and DW-MRS results for both samples.Discussion & Conclusion
Figure 3 and 4 show the DW fat peak signal decay curves, which are very similar when comparing the two AT samples with different adipocyte sizes. However, the decay of the ADC as a function of the diffusion time is different between the two samples and reveals different restrictions on the lipid diffusivity that can be employed to estimate the adipocyte size. Although white adipocytes are relatively large, the ex-vivo validation is associated with lower free lipid diffusivity compared to in-vivo conditions and the gradient strength of a clinical 3T system is limited, the DW-MRS-based adipocyte size measurement was feasible and matched histology.In conclusion, it was shown with preliminary data and for the first time that DW-MRS is capable of ex-vivo measuring human adipocyte size in white AT using clinical 3T system hardware. A limitation of the present study is the low number of measured samples. A validation study with a larger number of samples is currently ongoing. The translation of the method to the in-vivo application in white AT remains challenging due to the sensitivity of the technique to motion and the associated long scan time, and requires further developments because of its high relevance in the metabolic research community.Acknowledgements
We would like to thank Professor Rainer Burgkart for his help in the construction of the decoupling table as well as Dr. Ursula Schulze-Eilfing and Dr. Charlotte Kleeberger for sample preparation. The present work was supported by the European Research Council (grant agreement No 677661, ProFatMRI). This work reflects only the authors view and the EU is not responsible for any use that may be made of the information it contains. The authors acknowledge finally research support from Philips Healthcare.References
1. Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest 1973;52(4):929-941.
2. Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med 2008;59(6):1347-1354.
3. Lehnert A, Machann J, Helms G, Claussen CD, Schick F. Diffusion characteristics of large molecules assessed by proton MRS on a whole-body MR system. Magn Reson Imaging 2004;22(1):39-46.
4. Steidle G, Eibofner F, Schick F. Quantitative Diffusion Imaging of Adipose Tissue in the Human Lower Leg at 1.5 T. Magnetic Resonance in Medicine 2011;65(4):1119-1125.
5. Jezzard P, Barnett AS, Pierpaoli C. Characterization of and correction for eddy current artifacts in echo planar diffusion imaging. Magn Reson Med 1998;39(5):801-812.
6. Anderson. Analysis and correction of motion artifacts in diffusion weighted imaging. Magn Reson Med 1994;32(3):379-387.
7. Weidlich D, Honecker J, Gmach O, Wu M, Burgkart R, Ruschke S, Franz D, Menze BH, Skurk T, Hauner H, Kulozik U, Karampinos DC. Measuring large lipid droplet sizes by probing restricted lipid diffusion effects with diffusion-weighted MRS at 3T. Magn Reson Med 2019;81(6):3427-3439.
8. Cao P, Fan SJ, Wang AM, Xie VB, Qiao Z, Brittenham GM, Wu EX. Diffusion magnetic resonance monitors intramyocellular lipid droplet size in vivo. Magn Reson Med 2015;73(1):59-69.
9. Verma SK, Nagashima K, Yaligar J, Michael N, Lee SS, Xianfeng T, Gopalan V, Sadananthan SA, Anantharaj R, Velan SS. Differentiating brown and white adipose tissues by high-resolution diffusion NMR spectroscopy. J Lipid Res 2017;58(1):289-298.
10. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. Journal of Clinical Endocrinology & Metabolism 2007;92(3):1023-1033.
11. Ruschke S, Kienberger H, Baum T, Kooijman H, Settles M, Haase A, Rychlik M, Rummeny EJ, Karampinos DC. Diffusion-weighted stimulated echo acquisition mode (DW-STEAM) MR spectroscopy to measure fat unsaturation in regions with low proton-density fat fraction. Magn Reson Med 2016;75(1):32-41.
12. Mitra PP, Sen PN, Schwartz LM. Short-Time Behavior of the Diffusion-Coefficient as a Geometrical Probe of Porous-Media. Physical Review B 1993;47(14):8565-8574.
Figures