4677
Intranasal insulin administration in a rat model of neonatal hypoinsulinemic hyperglycemia
Ivan Tkac1, Kathleen Ennis2, William H Frey II3, and Raghavendra Rao2
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States, 3Center for Memory & Aging, HealthPartners Institute, St Paul, MN, United States
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States, 3Center for Memory & Aging, HealthPartners Institute, St Paul, MN, United States
Synopsis
Hyperglycemia (HG) is common in the neonatal period in extremely low gestational age infants. The purpose of this study was to investigate acute and long-term effects of intranasal insulin administration on hippocampal neurochemical profile in a rat model of human perinatal HG. Hypoinsulinemic HG was induced in neonatal rats by injecting streptozotocin on postnatal day P2. Hippocampal neurochemical profiles were assessed at P7 and P56. Preliminary in vivo 1H MRS data demonstrate the feasibility and efficacy of intranasal insulin administration for normalizing neurochemical homeostasis in the hippocampus exposed to transient hypoinsulinemic HG in the neonatal period.
INTRODUCTION
Hyperglycemia (HG, blood glucose >150 mg/dL) is common in the neonatal period in extremely low gestational age infants 1. HG is caused by insufficient production of insulin (hypoinsulinism) and its peripheral action. Hypoinsulinemic HG is associated with long-term impairments in hippocampal synaptogenesis and function in neonatal rats 2. Insulin signaling is critical for normal hippocampal synaptogenesis and function 3. Targeting hippocampal insulinergic pathways via systemic insulin administration is feasible, but can cause hypoglycemia in preterm infants 4. Conversely, intranasally administered insulin bypasses systemic circulation and achieves beneficial neurological effects without causing hypoglycemia or lowering brain glucose levels 5. Intranasal insulin has been proven safe and effective for improving cognitive function in human adults with hippocampal dysfunction due to aging, Alzheimer’s disease or trauma 6. The purpose of this study was to investigate acute and long-term effects of intranasal insulin administration on hippocampal neurochemical profile in a rat model of human extremely preterm infant-equivalent hypoinsulinemic HG.METHODS
Hypoinsulinemic HG was induced in neonatal rats (N = 12) by injecting streptozotocin (STZ; 80 mg/kg i.p.) on postnatal day (P) 2. STZ caused transient destruction of pancreatic β cells in neonatal rats and led to hypoinsulinemic HG from P3 to P6. The control group (N = 6) was injected with citrate buffer. Blood glucose was monitored daily from P3 to P6. HG group was randomized to receive human regular insulin 1.5 IU through each nostril twice daily from P3 - P6 (STZ + insulin group, N = 6) or no treatment (STZ group). In vivo 1H MR spectra were acquired from the hippocampus on P7 and P56. Spontaneously breathing animals were anesthetized with 1.0 – 1.5% isoflurane and the body temperature was maintained at 370C. MRS data were collected at 9.4T using FASTMAP shimming 7 and LASER localization sequence 8 (TE = 15 ms, TR = 5 s) combined with VAPOR water suppression 9. Metabolites were quantified using LCModel with the spectrum of fast relaxing macromolecules included in the basis set. Unsuppressed water signal was used as an internal reference assuming 88% and 80% brain water content for P7 and P56, respectively.RESULTS
Transient hypoinsulinemic HG (P3 – P6) was confirmed by increased blood glucose levels in the STZ groups [mean ± SD (mg/dL); Control, 128 ± 15; STZ, 279 ± 132; STZ + insulin, 274 ± 89]. The spectral quality consistently achieved in this study (Fig. 1) allowed a high precision in vivo neurochemical profiling. In vivo 1H MRS data acquired on P7, soon after transient hypoinsulinemic HG (P3 – P6), showed small but significant changes in creatine, glutamate, taurine and PCr/Cr ratio (Fig. 2). Although statistical significance was not reached for a wide range of metabolites due to small sample size, a very consistent pattern between control, STZ and STZ+insulin groups was observed for a number of metabolites (ascorbate, aspartate, glucose, glutamine, myo-inositol, phosphoethanolamine, total choline and total creatine). A significant difference at P56 was found only for phosphocreatine, glutamate and PCr/Cr ratio (Fig. 3). However, a consistent pattern between control, STZ and STZ+insulin groups was observed for several metabolites (alanine, ascorbate, creatine, GABA, glucose, glutathione, myo-inositol and taurine) also at P56.DISCUSSION
These preliminary data demonstrate the feasibility and efficacy of intranasal insulin administration for normalizing neurochemical homeostasis in the hippocampus exposed to transient hypoinsulinemic HG in the neonatal period. In addition, the neurochemical changes in the untreated STZ group suggest that short-duration (P3 – P6) hypoinsulinemic HG results in long-term changes in the hippocampal neurochemical profile. These neurochemical changes also indicate a much broader impact of transient neonatal hypoinsulinemic HG on the hippocampus. This is consistent with the vulnerability of the hippocampus during this developmental phase. In addition, these data are in agreement with previous data in human preterm infants and neonatal rat models showing long-term hippocampus-centered cognitive deficits following hypoinsulinemic HG in the neonatal period 2,10.CONCLUSION
Intranasal insulin administration may reduce the adverse effects of neonatal transient hypoinsulinemic HG on hippocampal development.Acknowledgements
Supported by NIH grants P41 EB027061 and NS076408 and by Viking Children’s Fund, Department of Pediatrics, University of Minnesota.References
1. Ramel SE, Long JD, Gray H, Durrwachter-Erno K, Demerath EW, Rao R. Neonatal hyperglycemia and diminished long-term growth in very low birth weight preterm infants. J Perinatol 2013; 33: 882-6. 2. Satrom KM, Ennis K, Sweis BM, Matveeva TM, Chen J, Hanson L, Maheshwari A, Rao R. Neonatal hyperglycemia induces CXCL10/CXCR3 signaling and microglial activation and impairs long-term synaptogenesis in the hippocampus and alters behavior in rats. J Neuroinflammation 2018; 15: 82. 3. De Felice FG, Benedict C. A Key Role of Insulin Receptors in Memory. Diabetes 2015; 64: 3653-5. 4. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med 2008; 359: 1873-84. 5. Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 2008; 9 Suppl 3:S5. 6. Benedict C, Frey WH 2nd, Schioth HB, Schultes B, Born J, Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp Gerontol 2011; 46: 112-5. 7. Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 2000; 43: 319-23. 8. Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 2001; 153: 155-77. 9. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999; 41: 649-56. 10. Gonzalez Villamizar JD, Haapala JL, Scheurer JM, Rao R, Ramel SE. Impact of neonatal hyperglycemia on body composition and neurodevelopmental outcomes on infants born less than 32 weeks gestation. Pediatric Academic Societies. Baltimore, MD 2019: Abstract no. 3169446.Figures
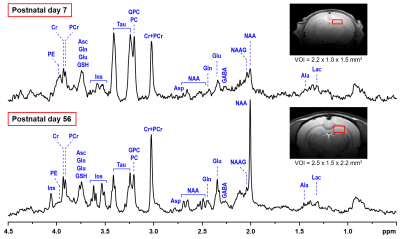
Representative in vivo 1H MR spectra acquired from the hippocampus STZ rat on
postnatal days 7 and 56. LASER localization sequence with VAPOR water suppression (TE = 15 ms, NT = 160). Axial FSE
images show the selection of a 3.3-μL (P7) and an 8.3-μL (P56) VOIs in the
dorsal hippocampus.

Comparison of hippocampal neurochemical profiles of control, STZ and
STZ+insulin rat groups (N = 6 per group) on postnatal day 7. Statistical
analysis: t-test, * p < 0.05, trend # p < 0.1. Error bars indicate SD.
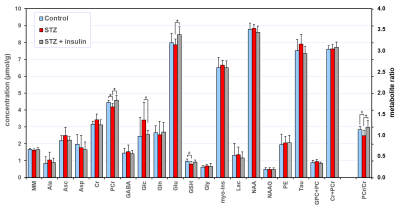
Comparison of hippocampal neurochemical profiles of control, STZ and
STZ+insulin rat groups (N = 6 per group) on postnatal day 56. Statistical
analysis: t-test, * p < 0.05, trend # p < 0.1. Error bars indicate SD.