4676
1H MRS of brain metabolism during cardiopulmonary bypass surgery in a neonatal pig model1Radiology, Stanford University, Stanford, CA, United States, 2Cardiothoracic Surgery, Stanford University, Stanford, CA, United States
Synopsis
Although cardiopulmonary bypass (CPB) surgery in the neonate carriers significant risk for long-term neuronal deficits, details of brain metabolism during varying flow conditions for CPB, such as deep hypothermic circulatory arrest (DHCA) versus antegrade cerebral perfusion (ACP), remain incompletely understood. Metabolic changes in the brain have been observed under different CPB conditions, but which are truly causative of neurotoxicity and injury remains unclear. In this preliminary preclinical study, brain metabolism, as assessed using 1H MRS, during DHCA is active and abnormal, resulting in a buildup of lactate and loss of energy substrates. ACP may prevent these abnormalities.
Introduction
Congenital heart disease affects nearly 1% of births in the US, with ~25% of these babies requiring surgery1. Unfortunately, more than half of those patients develop some form of neurological deficits2. Details regarding brain metabolism during varying flow conditions for cardiopulmonary bypass (CPB), including deep hypothermic circulatory arrest (DHCA) versus antegrade cerebral perfusion (ACP), remain incompletely understood. Metabolic changes in the brain have been observed under different CPB conditions,3,4 but which are truly causative of neurotoxicity and injury remains unclear. Here, we sought to assess dynamic brain metabolic changes during CPB surgery using 1H MRS5.Methods
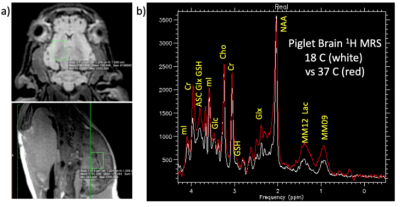
We conducted five CPB studies under DHCP (n=3) and ACP conditions (n=2), using a 3T GE MR750 MRI scanner (Waukesha, WI) to dynamically assess corresponding cerebral metabolic changes. Neonatal ~4kg piglets underwent median sternotomy and central cannulation for a 60-minutes period of CPB at 18°C under either DHCA or ACP as previously described6.Following rewarming to 37°C, MRI was discontinued and animals euthanized. The brains were then surgically removed and reviewed by a veterinary pathologist to confirm absence of gross pathology. Following anatomical MRI for voxel selection, proton MR spectra were repeatedly collected using either a single-voxel sLASER7 or a modified PRESS pulse sequence with the following acquisition parameters: TE/TE = 2000/35 ms, 15 x 12 x 12 mm3 voxel, 64 averages, 2:56 minute acquisition, acquired with either a 16-channel flex or an 8-channel knee RF coil. To minimize out of voxel artifacts, the PRESS sequence was modified to use a sensitive point FID along with a 16-step phase cycle8. Brain temperature was monitored via the chemical shift difference between water and NAA9.
Results
All spectra were preprocessed by adjusting for the temperature-dependent water shift, in combination with a water-referencing based artifact elimination10. The data were then analyzed using LCModel fitting software11and quantified based on an assumed brain total creatine concentration of 8 mM. Representative dynamic metabolic data are shown in Figures 1-3.Discussion
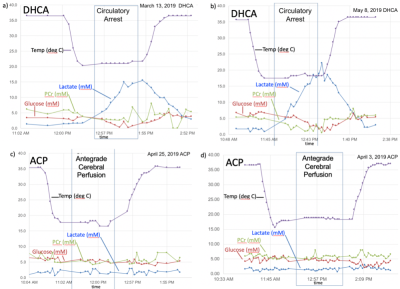
While difficult to directly compare these data sets due to varying acquisition parameters, several trends are evident. There is a marked buildup of brain lactate (estimated to increase at a rate of ~18 mM/hr) during DHCA, whereas no increased lactate is observed with ACP. This increase in lactate is consistent with prior microdialysis studies, however dynamic 1H MRS is more powerful in that the rate of lactate creation can be readily calculated. Furthermore, the increase in lactate is accompanied by a smaller, but measurable decrease (~ 3-4 mM/hr) in both brain glucose and PCr during DHCA, which is again absent with ACP. Interestingly, given each glucose molecule produces two lactate molecules during anaerobic metabolism, the rate of lactate creation outpaces the reduction in glucose, suggesting utilization of some other fuel source. The most likely hypothesis is that brain glycogen stores are also consumed during DHCA, although this has not been previously reported. Glycogen is known to play an important role in brain energy metabolism as well as memory formation12 with brain glycogen concentration reported to be ~3 mM13 and shown in neurons to significantly contribute to hypoxia tolerance14.In one DHCA animal, lactate production appeared to significantly decrease or even stop as glucose levels reach undetectable concentrations (ca 50 minutes post circulatory arrest), potentially representing a critical point of brain injury when energy production may have become exhausted.
These preliminary data also suggested a loss of brain Glx (glutamate + glutamine) during with hypothermia (see Fig. 3). There is some indirect support for such changes Henry et al. reported an 53% reduction in brain Glx in hibernating vs awake ground squirrels, which the authors attributed to decrease neuronal function15. An alternative hypothesis is that during hypothermia the brain shows an increase preference for Glx as fuel sources rather than glucose16. The catabolizing of Glx as fuel is supported by the spike in blood ammonia levels that we observed upon restarted circulation in the one animal for which these samples were available (see Fig 3-left). This finding may be of particular importance given ammonia is known to neurotoxic17 and blood ammonia has been shown to be predictive of neurologic outcome in cardiac patients treated with therapeutic hypothermia18.
Conclusions
Proton MR spectroscopy during bypass provides an unprecedented opportunity to monitor brain cellular metabolism in real time and clearly reveals marked metabolic changes during DHCA as compared to ACP. Non-invasive dynamic in vivo mapping of cerebral metabolism using MRS enables a more complete understanding of brain changes during cooling, hypothermia, and the recovery periods, offering significant advantages over other more invasive techniques. The preliminary results presented here suggest ACP may prevent the substantial buildup of brain lactate and loss of energy substrates that occurs with DHCA. Hence, 1H brain MRS of preclinical models may prove useful for determining optimal surgical parameters including body temperatures and ACP flow rates. Future developments include temperature-dependent spectral basis functions and T2 relaxation rates.Acknowledgements
NIH P41EB015891References
1. Oster ME, Kim CH, Kusano AS, Cragan JD, Dressler P, Hales AR, Mahle WT, Correa A. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113(6):1036-40.
2. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Jr., Li J, Smith SE, Bellinger DC, Mahle WT, Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143-72.
3. Mavroudis CD, Karlsson M, Ko T, et al. Cerebral mitochondrial dysfunction associated with deep hypothermic circulatory arrest in neonatal swine. Eur J Cardiothorac Surg. 2018;54:162-168.
4. Tang Z, Liang M, Chen G, et al. Neuroprotective effect of selective antegrade cerebral perfusion during prolonged deep hypothermic circulatory arrest: Cerebral metabolism evidence in a pig model. Anatol J Cardiol. 2018;19:2-10.
5. Hanley, F.L.I., H.; Hurd R. E.; Gu M.; Riemer, R. K.; Spielman, D.M. Comparison of dynamic brain metabolism during antegrade cerebral perfusion versus deep hypothermic circulatory arrest using proton MR spectroscopy. Journal of Thoracic and Cardiovascular Surgery JTCVS-19-2062R1, in press (2019).
6. Sasaki T, Tsuda S, Riemer RK, Ramamoorthy C, Reddy VM, Hanley FL. Optimal flow rate for antegrade cerebral perfusion. J Thorac Cardiovasc Surg. 2010;139(3):530-5; discussion 5. doi: 10.1016/j.jtcvs.2009.12.005.
7. Deelchand DK, Kantarci K, Oz G. Improved localization, spectral quality, and repeatability with advanced MRS methodology in the clinical setting. Magn Reson Med. 2018;79(3):1241-50.
8. Gu M HR, and Spielman D, editor. Quantification of Glutathione and Ascorbate in the Human Brain using Short-TE PRESS. ISMRM; 2019; Montreal, Canada.
9. Corbett RJ, Laptook AR, Tollefsbol G, Kim B. Validation of a noninvasive method to measure brain temperature in vivo using 1H NMR spectroscopy. J Neurochem. 1995;64:1224-1230.
10. Hurd R, Magnetic resonance spectroscopic imaging having reduced parasitic side band signals patent 6,069,478. 1997.
11. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260-4.
12. Waitt AE, Reed L, Ransom BR, Brown AM. Emerging Roles for Glycogen in the CNS. Front Mol Neurosci. 2017;10:73. Epub 2017/04/01. doi: 10.3389/fnmol.2017.00073. PubMed PMID: 28360839; PMCID: PMC5352909.
13. Oz G, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658-79.
14. Saez I, Duran J, Sinadinos C, Beltran A, Yanes O, Tevy MF, Martinez-Pons C, Milan M, Guinovart JJ. Neurons have an active glycogen metabolism that contributes to tolerance to hypoxia. J Cereb Blood Flow Metab. 2014;34(6):945-55.
15. Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures: in vivo (1)H MRS study of a hibernating mammal. J Neurochem. 2007;101(6):1505-15.
16. Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, Rutter J, Merritt ME, DeBerardinis RJ. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56(3):414-24.
17. Albrecht J, Zielinska M, Norenberg MD. Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem Pharmacol. 2010;80(9):1303-8.
18. Cho YM, Lim YS, Yang HJ, Park WB, Cho JS, Kim JJ, Hyun SY, Lee MJ, Kang YJ, Lee G. Blood ammonia is a predictive biomarker of neurologic outcome in cardiac arrest patients treated with therapeutic hypothermia. Am J Emerg Med. 2012;30(8):1395-401.
Figures