4674
Fixel-based analysis reveals white matter changes following balance training in young brain injured patients: A longitudinal study1Mary Mackillop Institute for Health Research, Australian Catholic University, Melbourne, Australia, 2Florey Institute of Neuroscience and Mental Health, University of Melbourne, Melbourne, Australia
Synopsis
Traumatic brain injury (TBI) is one of the leading causes of death and disability in children and adolescents. Young TBI patients suffer from gross motor deficits, such as postural control deficits, which can severely compromise their daily life activities. Training programs have shown behavioral improvement; evidence of changes in WM morphology, however, has not been clear. We employ a fixel-based analysis (FBA) to investigate whether balance training results in significant changes of WM organization across whole brain in young TBI patients over time. Our results have shown that balance training induced signigicant macrostructural white matter changes (i.e. log-FC & FDC).
INTRODUCTION
Traumatic brain injury (TBI) is one of the leading causes of death and disability in children and adolescents. Young TBI patients suffer from gross motor deficits, such as postural control deficits, which can severely compromise their daily life activities. Our previous research has demonstrated a strong relationship between alterations in white matter (WM) organization, structural brain networks and postural control deficits1,2. Various task-specific training programs have been utilized to improve motor functioning in TBI patients3, which have shown behavioural improvement; evidence of changes in WM morphology induced by training is less clear4. Presumably, this conclusion drawn from previous studies could be potentially deviated by the inherent limitations of diffusion tensor analysis in detecting WM changes5.In this study, we employ a fixel-based analysis (FBA)6 to investigate whether balance training results in significant changes of WM organization across whole brain in TBI patients over time. The advantages of applying FBA in this TBI study include: (1) more fibre-specific metrics are used (metrics associated with microstructural & macrostructural WM changes) as compared to diffusion tensor metrics (such as mean diffusivity and fractional anisotropy)7; (2) group differences are detected from whole brain instead of only focusing on certain brain regions-of-interest7.
METHODS
(1) Subjects and training: 17 moderate to severe TBI patients (9 females; mean age = 15±3 years) and 17 typically developing subjects (10 females; mean age = 15±2 years). Both groups attended a home-based balance training program for 8 weeks, with 5 sessions per week (~30 min/session)7.(2) Assessment: Participants underwent MRI scans at 3 time points: (a) pre-test: prior to training; (b) post-test: after 8 weeks of training (mean =57±2days).
(3) MRI data acquisition: Diffusion-weighted images (TR/TE=8000/91ms, voxel size: 2.2mm isotropic, 60 contiguous slices, b=1000s/mm2 at 64 diffusion gradient directions, along one b=0 s/mm2 volume) and T1-weighted images (TR=2300ms, TE=2.98ms, voxel size: 1x1x1.1 mm3, FOV=256x240, 160 contiguous sagittal slices) were collected on a 3T Siemens Trio scanner.
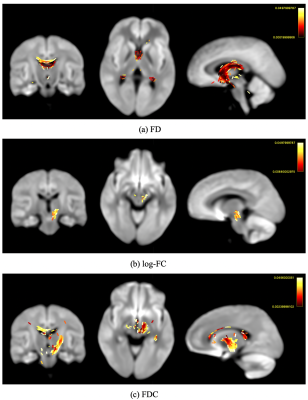
(4) Fixel-based analysis6: FBA was performed using MRtrix38 to detect white matter changes between: (i) TBI and healthy control groups; (ii) pre-test and post-test groups using the following steps: (a) Data pre-processing; (b) A unbiased group fibre orientation distribution (FOD) template was generated using 26 subjects (13 from each group); (c) Whole-brain tractography was conducted on the group FOD template with 20 million tracts generated, which was filtered down to 2 million by applying spherical-deconvolution informed filtering of tractograms (SIFT)9; (d) Three quantitative FBA metrics were calculated: (i) Fibre density (FD): measuring fibre density of specific white matter fibre bundles within voxels; (ii) Fibre-bundle cross-section in logarithm scale (log-FC): measuring macrostructural change of a fibre bundle; and (iii) a combined measure of a fibre density and cross-section (FDC).
(5) Statistical analyses: Statistical inference was performed using the connectivity-based fixel enhancement approach10. Specifically, two-sample t-test was applied for the pre-test analysis, and a paired-sample t-test was conducted to detect training effect on FBA metrics. (a) FBA results at baseline were generated using 5000 permutations and family-wise error (FWE)-corrected statistical p<0.05 is considered significant; (b) If no significant changes are detected after training, the prior knowledge of susceptible fibers from the baseline group analyses is obtained to increase the sensitivity for detecting training effects. Practically, the small-template correction (STC) is performed as follows: (i) A small template for each FBA metric is created by thresholding (p<0.01, retaining ~1/15 of total number of whole-brain fixels) uncorrected p-value map of same FBA metric from baseline results; (ii) statistical analysis is then performed within the small template.
RESULTS
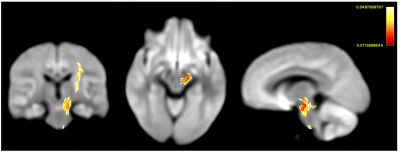
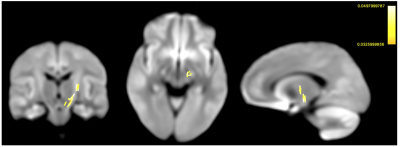
Comparing groups at pre-test, Figures 1 shows streamline segments in which associated fixels had significantly decreased values in all 3 FBA metrics (FWE-corrected p-value<0.05). Specifically, lower FD is found mainly in fornix (Figure 1 (a)) and reductions of cross-section are detected in superior cerebellar peduncles (Figure 1(b)); decreases of FDC distributed corpus collosum, thalamus, and superior cerebellar peduncles (Figure 1(c)).No significant white matter changes following balance training have been detected following strict whole-FBA FWE correction. However, using the small-template correction, our results demonstrate significantly increased log-FC and FDC after receiving balance training (Figure 2 & 3). In addition, the enhancement of both FBA metrics consistently locates in superior cerebellar peduncles. Further inspection reveals that enhanced FBA metrics occur around the same areas that were shown to have reductions for both metrics. Nevertheless, no significant changes in FD following balance training could be observed.
DISCUSSION
In this study, a longitudinal study that investigate balance training effect in young TBI patients has been conducted using an advanced MRI analysis approach, fixel-based analysis.Using FBA, our longitudinal results showed that balance training induced significant white matter changes in log-FC and FDC. These significant fixels coincide with those that were impaired in TBI group during pre-test, suggesting a sign of white matter recovery. Taking together, these findings suggest that balance training contributes mainly to macrostructural (i.e. log-FC & FDC) instead of microstructural changes (i.e. FD). Therefore, this approach should provide insights into the underlying mechanisms of rehabilitative training.
Acknowledgements
No acknowledgement found.References
1. Caeyenberghs K, Leemans A, Geurts M, Taymans T, Linden CV, Smits-Engelsman BC, Sunaert S, Swinnen SP. Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Human Brain Mapping. 2010; 31(7):992-1002.
2. Caeyenberghs K, Leemans A, De Decker C, Heitger M, Drijkoningen D, Linden V, Sunaert S, Swinnen SP. NeuroImage: Clinical. 2012; 1(1): 106-115.
3. Caeyenberghs K, Clement A, Imms P, Egan G, Hocking DR, Leemans A, Metzler-Baddeley C, Jone DK, Wilson PH. Evidence for training-dependent structural neuroplasticity in brain-injured patients: A critical review. Neurorehabil Neural Repair. 2018; 32(2): 99-114.
4. Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM. White matter involvement after TBI: Clues to axon and myelin repair capacity. Experimental Neurology. 2016; 275(3): 328-33.
5. Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2012; 73:239-54.
6. Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A. Investigating white matter fiber density and morphology using fixel-based analysis. NeuroImage. 2017: 144(A): 58-73. 7. 7. Drijkoningen D, Caeyenberghs K, Leunissen I, Vander Linden C, Leemans A, Sunaert S, Duysens J, Swinnen SP. Neuroimage Clin. 2014; 7:240-51.
8. Tournier JD, Smith RE, Raffelt D, Tabbara R, Dhollander T, Christiaens D, Pietsch M, Jeurissen B, Yeh CH, Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 2019; 202: 116-37.
9. Simth RE, Donald JD, Calamante F, Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. NeuroImage. 2012; 67: 298-312.
10. Raffelt DA, Smith RE, Ridgway GR, Tournier JD, Vaughan DN, Rose S, Henderson R, Connelly A. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measure in the presence of crossing fibres. NeuroImage. 2015; 117:40-55.
Figures