4673
Early Changes in Diffusion Tensor Metrics between Different Final Damage Outcomes after Experimental Neonatal Hypoxic Ischemia1Neuroscience Research Center, Taipei Medical University, Taipei, Taiwan, 2Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, 3Translational Imaging Research Center, Taipei Medical University Hospital, Taipei, Taiwan, 4Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, 5Department of Biomedical Imaging and Radiological Science, China Medical University, Taichung, Taiwan, 6Department of Pediatrics, College of Medicine, National Cheng Kung University, Tainan, Taiwan, 7Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan
Synopsis
Changes in diffusion tensor metrics within 6 h after hypoxic ischemia (HI) in different brain regions in neonatal rats is associated with the final lesion severity after hypoxic ischemia. We also demonstrated that the early changes within 6 h after HI may also correlate to the alteration in ultra-structure in the neurons and axons following the injury.
Introduction
Neonatal hypoxic-ischemia (HI) trigger cascades of evolving neurotoxic events within hours that last for days to weeks after birth and evolve into HI encephalopathy.1, 2 An early biomarker indicating the severity of HI before hypothermia practice is emerging to stratify the neonates for the following treatment. While particular injury pattern on MRI may correlate to motor and/or cognitive deficit during development3, 4, the injury is quite difficult to define unless devastating. By using the Rice-Vannucci model with different severity of HI5, we demonstrated that the early changes in micro- and meso-scopic structure depicted by transmission electric microscopy (TEM) and diffusion tensor MRI within 6 h after HI insult is associated to the final neurodevelopmental outcomes.Methods
Seventeen Sprague-Dawley rat pups on postnatal day 10 were anesthetized with 1-2% isoflurane, following by permanent ligation of the right common carotid artery with 5-0 surgical silk to induce HI injury with Rice-Vannucci model. After surgery, the pups returned to their dams for 1-hour recovery before 2.5 hours of hypoxia. During hypoxia, the pups were placed in air-tight 500-ml containers with 37°C humidified 8% oxygen (balance, nitrogen). Animal MR Imaging data were acquired within 6 h after hypoxia on a Brucker 7T PharmaScan scanner. Animals were anesthetized under 0.75 % isoflurane, and the stereotaxic headpiece and holder consisting of ear and tooth bars will be used to immobilize the head. Diffusion data and anatomical data were acquired in the acute and at 7 days post HI as described previously.6 A subset of four male pups after HI were sacrificed within 6 h to examine ultrastructural changes using transmission electric microscopy (TEM).7 Other rats were sacrificed after the acquisition of T2-weighted images at 7 days after HI insult. The gross neuropathological examination was performed to categorize the brain injury based on the atrophy with cystic cavitation in the ipsi-lesional hemisphere.8 The ADC-deficit area was identified as regions showing ADC < 70% of the contralateral homologous brain.6, 9, 10 The DTI metrics including ADC, fractional isotropy (FA), axial diffusivity (AD) and radial diffusivity (RD)11 in our interested region of interest (ROIs) including the corpus callosum (c.c.), cortex, hippocampus, lateral thalamus (LT) and ventral thalamus (VT) were confined within the acute ADC-deficit area and matched with neonatal brain atlas. The brain damage outcome is defined as the volume of the ipsilateral hemisphere or ROIs divided by that of the contralateral hemisphere or ROIs at 7 days post HI injury. Significant level was set at P<0.05. Error bars were STD.Results & Discussion
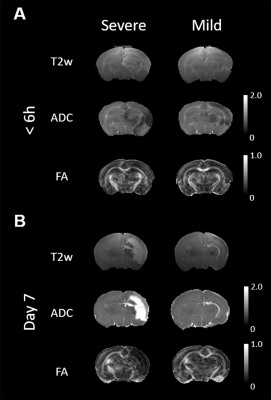
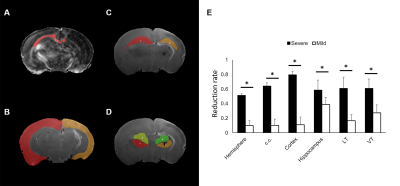
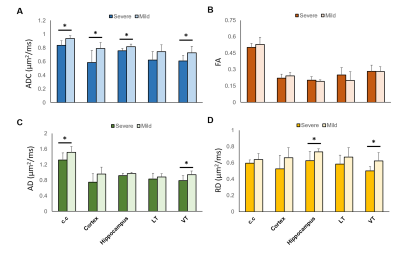
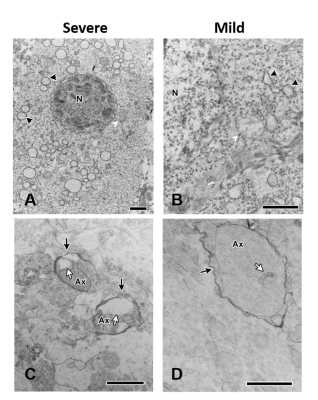
We have demonstrated a spectrum of brain damage outcome severity after HI in neonatal animals with Rice-Vannucci model.6 The HI injury was soon depicted by ADC deficit in the ipsilateral cortex, hippocampus, thalamus, etc. within 6 h after insult (Figure 1A). Significant hemispheric volume change was observed at day 7 post HI to classify the outcome severity (Figure 1B).6 In addition to the significant hemispheric reduction rate, prominent brain regional reduction was observed after ROI analysis (Figure 2A-D). In contrast to most dramatic reduction rate between mild and severe outcome in the cortex, the hippocampus showed relative little difference between the mild (38.5±10.1 ) and severe (58.8±13.6 ) outcome group (Figure 2E). We observed the change of tensor metrics between different outcome groups in some brain regions within 6 h after HI. Significant lower mean ADC value were observed in multiple brain regions including the corpus callosum, hippocampus and ventral thalamus in the severe outcome group (P<0.05, Figure 3A). While no difference was shown in FA value between groups (Figure 3B), significant lower AD in the corpus callosum and ventral thalamus (P<0.05, Figure 3C) and lower RD in the hippocampus and ventral thalamus (P<0.05, Figure 3D) was found in the severe outcome group. By using TEM, marked apoptotic-like degenerating neurons (Figure 4A) were found in the cortical area with significant ADC deficit changes within 6 hours after HI. Prominent myelin loss and axon detraction were observed in the white matter areas in both groups of animals (Figure 4C and D). Future studies will attempt to assess the correlation of different diffusion tensor metrics for early prediction of susceptibility in regional HI outcome.Acknowledgements
This study was funded in part by Ministry of Science and Technology (MOST 108-2314-B-038-002), Taipei, Taiwan.References
1. Douglas-Escobar M and Weiss M D, Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr, 2015. 169(4): 397-403.
2. Johnston M V, Fatemi A, Wilson M A, et al., Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol, 2011. 10(4): 372-82.
3. Chau V, Poskitt K J, and Miller S P, Advanced neuroimaging techniques for the term newborn with encephalopathy. Pediatr Neurol, 2009. 40(3): 181-8.
4. Miller S P, Ramaswamy V, Michelson D, et al., Patterns of brain injury in term neonatal encephalopathy. J Pediatr, 2005. 146(4): 453-60.
5. Rice J E, 3rd, Vannucci R C, and Brierley J B, The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol, 1981. 9(2): 131-41.
6. Kao Y C J L, C.F.; Huang, C.C. Chen, C.Y., Early Apparent Diffusion Coefficient Deficit Correlates to Final Outcome in Experimental Neonatal Hypoxic Ischemia. ISMRM Preceeding, 2018.
7. Hsu Y C, Chang Y C, Lin Y C, et al., Cerebral microvascular damage occurs early after hypoxia-ischemia via nNOS activation in the neonatal brain. J Cereb Blood Flow Metab, 2014. 34(4): 668-76. 8. Lee H T, Chang Y C, Tu Y F, et al., VEGF-A/VEGFR-2 signaling leading to cAMP response element-binding protein phosphorylation is a shared pathway underlying the protective effect of preconditioning on neurons and endothelial cells. J Neurosci, 2009. 29(14): 4356-68.
9. Kao Y J, Oyarzabal E A, Zhang H, et al., Role of Genetic Variation in Collateral Circulation in the Evolution of Acute Stroke: A Multimodal Magnetic Resonance Imaging Study. Stroke, 2017. 48(3): 754-761.
10. Kuo D P, Lu C F, Liou M, et al., Differentiation of the Infarct Core from Ischemic Penumbra within the First 4.5 Hours, Using Diffusion Tensor Imaging-Derived Metrics: A Rat Model. Korean J Radiol, 2017. 18(2): 269-278.
11. Kao Y J, Lui Y W, Lu C F, et al., Behavioral and Structural Effects of Single and Repeat Closed-Head Injury. AJNR Am J Neuroradiol, 2019.
Figures