4666
Unveiling early cortical and white-matter maturation and the effect of music in preterm brain development: a longitudinal fixel-based analysis1Department of Women-Children-Teenagers, Hôpitaux Universitaires de Genève, Geneva, Switzerland, 2Department of Radiology and Medical Informatics, CIBM, University of Geneva, Geneva, Switzerland
Synopsis
Dynamic brain macrostructural and microstructural changes occur from mid-fetal stage to birth. Prematurity disrupts brain maturation during this critical period and music might enhance activity-dependent-plasticity during early brain development. Using a longitudinal fixel-based analysis, we evaluated preterm brain macro and microscopic fiber changes from 33 weeks to term-age and the impact of music, during neonatal unit stay, on these changes. We show that fiber density (FD) and fiber-bundle cross-section (FC) increase in all major white-matter fibers over time, while in the cortex FD decreases and FC increases. Music intervention lead to a significant cortical FC increase in comparison to standard-of-care.
INTRODUCTION
Dynamic macrostructural and microstructural changes take place from mid-fetal stage to birth, namely grey-matter pruning and white matter (WM) volume expansion driven by increasing axon diameter, density and mydlination.1-3 Preterm birth disrupts brain maturation through exposition to different noxious stimuli and deprivation from meaningful sensory inputs during a critical period of development.4,5 Musicotherapy has been used as an approach relevant for activity-dependent brain plasticity and might influence networks formed early in development and affected by prematurity.6-9 Using fixel-based analysis (FBA)10, we studied preterm brain fiber microscopic density and macroscopic morphology changes between 33 weeks of gestational age (wGA) and term-equivalent age (TEA). Furthermore, we assessed the longitudinal effect of a music intervention during neonatal stay on the observed significant structural brain maturational changes.METHODS
38 very preterm newborns (VPT, <32 wGA), 17 without music exposure and 21 exposed daily to music during neonatal stay (starting at 33wGA), underwent an MRI (3T Siemens Prisma) at two time-points, 33wGA and TEA, including multi-shell diffusion imaging (MSDI) (TE=85ms, TR=3170ms, voxel size 1.8x1.8x1.8mm3, multi-band acceleration factor 2; 4 volumes without diffusion-weighting; 10 non-collinear directions with b=200 s/mm2, 30 non-collinear directions with b=1000 s/mm2; 50 non-collinear directions with b=2000 s/mm2) and T2-weighted image (TE=160ms, TR=4990ms, voxel size 0.8x0.8x1.2mm3) acquisitions. Diffusion data were preprocessed following FBA recommended pipeline,10 using MRtrix311 for denoising, Gibbs ringing removal, bias field corrections and intensity normalisation and FSL v5.0.10 diffusion toolbox12,13 for distortions caused by motion and eddy currents, using EDDY function adapted for neonatal data.14,15 Data were up-sampled to 1.3 mm3 isotropic voxel size, fiber orientation distribution (FOD) was estimated in each voxel using multi-tissue constrained spherical deconvolution, and FOD maps were computed for each subject using a group-average response function. An unbiased study-specific FOD template was computed using 18 intra-subject templates (10 VPT with and 8 without music exposure). First, all FOD maps at 33wGA were averaged to generate a time-point 1 template (t1), whereas all FOD maps at TEA were averaged to generate a time-point 2 (t2) template. These two templates were brought to the midway-space. The FOD maps from subjects at 33wGA were registered to t1 template in midway-space, whereas FOD images from subjects at TEA were registered to t2 template in midway-space. These registered FOD maps at the two time-points of each subject were averaged to generate the intra-subject templates, which were subsequently averaged to create the final population template. Each subject FOD map was registered to this template, where whole-brain tractography was performed generating 20 million tracts using Mrtrix3 iFOD2 algorithm and then filtered to 2 million tracts using spherical-deconvolution informed filtering of tractograms (SIFT). Brain maturational changes between 33wGA and TEA were estimated using connectivity-based fixel enhancement16 analysis with 5000 permutations and family-wise error for multiple comparisons correction, performed for each FBA output metric: FD, log(FC), and fiber density and cross-section (FDC). To assess the effect of the music intervention, the brain regions containing the fixels where FBA output metrics were statistically significantly different (pFWE<0.05) between the two time-points were used as fixel masks (Fig. 1 and 2). The FBA output metrics were calculated using these masks superposed to the population template for each participant at each time-point. The difference between the two time-points per subject was accessed and a t-test of independent samples was performed to evaluate the mean change of each metric over time.RESULTS AND DISCUSSION
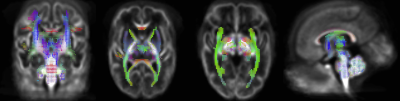
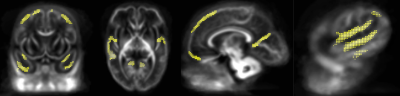
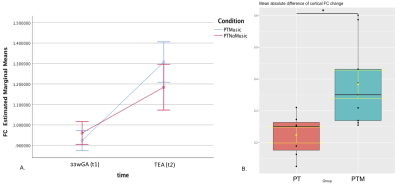
FBA of VPT at TEA, in comparison to 33wGA, revealed a statistically significant increase (pFWE<0.05) of FD, FC and FDC in all major WM fiber-bundles, including body, splenium and genu of corpus callosum, forceps major, forceps minor, anterior commissure, bilateral fornix, cingulum, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, cortico-spinal tract, internal and external capsules, optic radiations and right acoustic radiations, as well as thalamus, pons and cerebellum (Fig.1). These results support an increased WM maturation with GA, driven by the expected axonal number and diameter increase, as well as fiber-bundle diameter augmentation, due to pre-myelination. Additionally, in comparison to 33wGA, VPT at TEA presented a decreased FD and FDC, but increased FC (pFWE<0.05) in cortical areas, namely bilateral dorsal superior frontal gyrus, orbito-frontal cortex, bilateral superior and inferior temporal gyrus, right middle temporal gyrus, calcarine sulcus and right supra-marginal gyrus (Fig.2). Given the known cortical organization, these results suggest an increased cortical maturation with GA: the augmentation of cellular complexity with radial and tangential fibers may justify the decreased FD while the growth and complexity in cortical network may lead to the FC increase. Furthermore, using a fixel mask containing the previously described cortical regions, we found that VPT exposed to music had a significantly superior FC increase in these areas over time, compared to those not exposed to music, t(14)=2.898, p=0.012, (Fig.3), supporting a structural effect of music intervention in VPT brain cortical development.CONCLUSION
Overall, VPT brain undergoes important structural maturation between 33wGA and TEA, comprising an augmentation of FD, FC and FDC in all major WM fibers, thalamus, pons and cerebellum, as well as a decrease of FD, FDC with FC increase in the cortex. Early music intervention during neonatal stay lead to a significant cortical FC increase, in comparison to VPT receiving standard-of-care.Acknowledgements
The authors thank all clinical staff, namely in neonatology and unit of development of HUG Pediatric Hospital, all parents and newborns participating in the project, the Pediatrics Clinic Research Platform and the Center for Biomedical Imaging (CIBM) of the University Hospitals of Geneva, for all their help and support. This study was supported by the Swiss National Science Foundation n°324730_163084/1 and n°320030B_182832/1.References
1. Mills KL, Goddings AL, Herting MM, et al. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage 2016; 141: 273-81.
2. Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nature Reviews Neuroscience 2005; 6(12): 955-65.
3. Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20-45 weeks' gestation. Semin Fetal Neonatal Med 2006; 11(6): 415-22.
4. Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev 2005; 4(2): 271-87.
5. Kiss JZ, Vasung L, Petrenko V. Process of cortical network formation and impact of early brain damage. Curr Opin Neurol 2014; 27(2): 133-41.
6. Koelsch S, Kasper E, Sammler D, Schulze K, Gunter T, Friederici AD. Music, language and meaning: brain signatures of semantic processing. Nature neuroscience 2004; 7(3): 302-7.
7. Popescu M, Otsuka A, Ioannides AA. Dynamics of brain activity in motor and frontal cortical areas during music listening: a magnetoencephalographic study. NeuroImage 2004; 21(4): 1622-38.
8. Koelsch S. Towards a neural basis of music-evoked emotions. Trends in cognitive sciences 2010; 14(3): 131-7.
9. Zatorre RJ, Peretz I, Penhune V. Neuroscience and Music ("Neuromusic") III: disorders and plasticity. . Annals of the New York Academy of Sciences 2009; 1169: 1-2.
10. Raffelt DA, Tournier JD, Smith RE, et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 2017; 144: 58-73.
11. Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019; 202: 116137.
12. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208-S19.
13. Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50(5): 1077-88.
14. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125: 1063-78.
15. Bastiani M, Andersson JLR, Cordero-Grande L, et al. Automated processing pipeline for neonatal diffusion MRI in the developing Human Connectome Project. Neuroimage 2018.
16. Raffelt DA, Smith RE, Ridgway GR, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 2015; 117: 40-55.
Figures