4661
Low Frequency Fluctuations in White Matter of Perinatally HIV-Infected Adult Youths: Glial Cycling for Neuroplasticity and Repair?1Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2UCLA School of Nursing, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 3Pediatrics, Harbor-UCLA Medical Center, Torrance, CA, United States, 4The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA, United States, 5Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 6Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 7Infectious Diseases-Pediatrics, Miller Children’s Hospital of Long Beach, Long Beach, CA, United States, 8Pediatrics, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 9Pediatric, Keck School of Medicine of University of Southern California, Los Angeles, CA, United States, 10Children’s Hospital Los Angeles, Los Angeles, CA, United States, 11Radiology, Los Angeles County Harbor- UCLA Medical Center, Torrance, CA, United States, 12Biomedical Engineering, New Jersey Institute of Technology, Newark, NJ, United States
Synopsis
We evaluated the functional brain activity in perinatally HIV-infected youth (PHIVY) on cART by quantifying the amplitude of low frequency fluctuations (ALFF) and correlated with clinical and cognitive measures. We observed higher neural activity for ALFF in PHIVY compared to control. ALFF values in the cerebral white matter were positively correlated with viral load. Higher neural activity was associated with poorer performance in psychomotor function, abstract thinking, and social cognition. The findings suggest that long-term consequence of higher neuroinflammation and associated neurorepair in PHIVY may have a significant impact on regional spontaneous neuronal firing consequently impacting neurodevelopment and cognitive functioning.
Introduction:
In perinatally HIV-infected (PHIV) children, neurodevelopment occurs in the presence of HIV-infection. In addition, despite the success of combination antiretroviral therapy (cART), the brain can be a reservoir for latent HIV1-3. Consequently, patients often demonstrate long-term cognitive deficits and developmental delay5-8, which may be reflected in altered functional brain activity. However, there are limited studies on brain functional activity alteration in PHIV children9-11, and mostly are in younger populations. The purpose of the current study was to examine the functional brain activity by quantifying the amplitude of low frequency fluctuations (ALFF) in perinatally HIV-infected youth (PHIVY) on cART. Further, we investigated the relationship of ALFF changes with neuropsychological test results and measures of immune health such as CD4 count and viral load in the HIV-infected youths.Materials and Methods:
Eleven PHIVY (age 22.50±2.9 years, 8 females) and sixteen healthy controls (HC) (age 22.45±3.0 years, 9 females) participated in this study. All data were collected on a Siemens 3T Prisma MRI scanner using a 16 channel phased-array head ‘receive’ coil. Resting state functional MRI (rs-fMRI) scans were collected using an EPI sequence with: TR/TE=2000/27 ms, Flip angle=900, 40 slices, matrix=64x64; FOV=240 mm2; voxel-size=3.75x3.75x4 mm3; and 180 volumes/scan. To facilitate EPI distortion correction, a field map was acquired before the rs-fMRI scan: TR=430 ms, TE=7.35/9.81 ms, matrix=64x64, FOV=192 mm, 40 4mm slices, no gap. During the data acquisition, subjects were instructed to stare at a spot with their eyes open. In addition, high-resolution anatomical T1-weighted MPRAGE was acquired for registration and overlay of brain activity. For PHIVY subjects, additional clinical data were collected including HIV viral load, highest known viral load, CD4 T-cell counts, lowest known CD4, lowest known CD4%, and presence of HIV encephalopathy diagnosis. In addition, a comprehensive neuropsychological (NP) assessment battery was administered to all participants and grouped into 12 cognitive domains. Raw data and Z-scores were transformed into T-scores by utilizing established normative data. Domain T-scores were calculated by averaging the T-score of the individual tests comprising the neurocognitive domain.We used the SPM1212, DRIFTER13, DPABI14 and MATLAB (Mathworks Inc., Natick, MA) for data processing and analysis as described in detail elsewhere12-15. We used DRIFTER correction for physiological artifacts, and DPABI to conduct functional segregation of ALFF resting activity, and Regional Homogeneity (ReHo) for extracting information on the function of specific brain regions. Voxel-based comparison for group differences was performed using two sample t-test. For the regions that showed significant group differences, we used intrinsic masking in SPM to correlate the rs-fMRI data in the PHIVY to the neurocognitive measures and clinical parameters. The significance level was p =0.01 with cluster size ≥ 3.
Results:
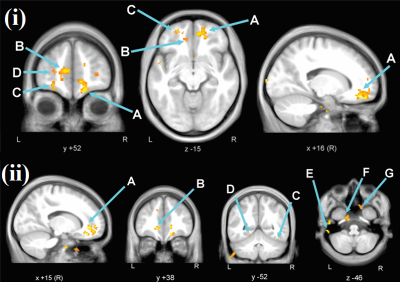
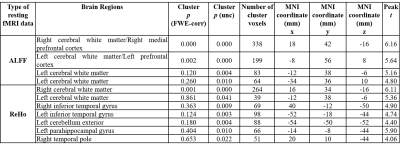
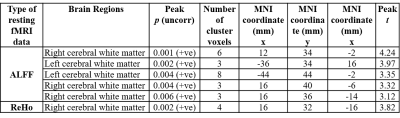
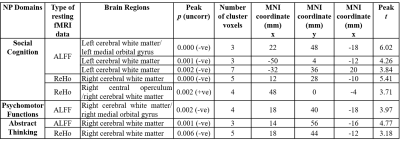
The patient and HC groups were not significantly different in age, gender. We found significantly higher regional activity in the ALFF in bilateral cerebral white matter (WM), and ReHo in bilateral cerebral white matter and bilateral inferior temporal gyrus, right temporal pole, and left parahippocampal gyrus and left cerebellar exterior for PHIVY compared to HC with age and gender as co-variates (Figure 1(i) & (ii)). Brain regions showing higher ALFF resting fMRI activity and higher ReHo resting state fMRI activity in PHIVY compared to HC are shown in Table 1. Higher ALFF and ReHo in PHIVY were associated with higher log10 viral load (Table 2) in the brain regions that show changes in ALFF and ReHo in the group analysis. Reductions in social cognition and abstract thinking in PHIVY were correlated with higher ALFF and ReHo (Table 3) after having masked to the brain regions that show significantly higher ALFF and ReHo. Table 3 also shows positive correlation of social cognition with ReHo and negative correlation of psychomotor functions with ALFF magnitude.Discussion:
We observed higher neural activity for low frequency oscillations in PHIVY compared to controls. We found a group of voxels in cerebral WM in the medial orbital gyrus consistently showing higher ALFF and ReHo for PHIVY compared to controls even after removal of local physiological artifacts. However, after removing global effects we did not see any significant ReHo activity reflecting the observed higher spontaneous neuronal firing to be WM specific. Previous DTI and morphological findings showed compromised WM integrity consistent with the current results16,17. Microglial activation has been associated with neuroinflammation18 and it has been shown that this particular sympathetic response of higher microglial activation is correlated with higher ALFF19. These data suggests possible higher neuro-inflammation in PHIVY in the presence of the HIV and cART.We found that ALFF values in the celebral WM were positively correlated with viral load. We observed that higher neural activity was associated with poorer performance in psychomotor function, abstract thinking, and social cognition adding to the growing body of evidence20,21 that suggests HIV-related cognitive impairment remains frequent in treated PHIVY.
Conclusion:
In summary, we observed significantly altered ALFF in cerebral white matter in PHIV-infected youth. These changes were correlated with poorer cognitive functions. The current findings suggest that long-term consequence of higher neuroinflammation and associated neurorepair in perinatally HIV infected may have a significant impact on regional spontaneous neuronal firing, potentially affecting neurodevelopment and cognitive functioning.Acknowledgements
This research was supported by the NIH grant: 1R21NS090956-01A1.References
1. Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–185.
2. Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300.
3. Lee GM, Gortmaker SL, McIntosh K. Quality of life for children and adolescents: impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117(2):273-283.
4. Patel K, Ming X, Williams PL, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials 219/219C Study Team Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23(14):1893–1901.
5. Martin SC, Wolters PL, Toledo-Tamula MA, et al. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART). Dev Neuropsychol. 2006;30(2):633–657.
6. Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603.
7. Smith R, Malee K, Leighty R, et al. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics. 2006;117(3): 851–862.
8. Nagarajan R, Sarma MK, Thomas MA, et al. Neuropsychological function and cerebral metabolites in HIV-infected youth. J Neuroimmune Pharmacol. 2012;7(4):981-990.
9. Herting MM, Uban KA, Williams PL, et al. Default Mode Connectivity in Youth With Perinatally Acquired HIV. Medicine (Baltimore). 2015;94(37):e1417.
10. Toich JTF, Taylor PA, Holmes MJ, et al. Functional Connectivity Alterations between Networks and Associations with Infant Immune Health within Networks in HIV Infected Children on Early Treatment: A Study at 7 Years. Front Hum Neurosci. 2018;11:635.
11. Yadav SK, Gupta RK, Hashem S, et al. Changes in resting-state functional brain activity are associated with waning cognitive functions in HIV-infected children. Neuroimage Clin. 2018;20:1204-1210.
12. Friston KJ. Statistical Parametric Mapping: the Analysis of Functional Brain Images. Elsevier/Academic Press, 2007.
13. Särkkä S, Solin A, Nummenmaa A, et al. Dynamic retrospective filtering of physiological noise in BOLD fMRI: DRIFTER. Neuroimage. 2012;60(2):1517-27.
14. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. , 2016;14(3):339-351.
15. Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22(1):360–366.
16. Sarma MK, Nagarajan R, Keller MA, et al. Regional brain gray and white matter changes in perinatally HIV-infected dolescents. Neuroimage Clin. 2013;4: 29-34.
17. Sarma MK, Keller MA, Macey PM, et al. White matter microstructure among perinatally HIV-infected youth: a diffusion tensor imaging study. J Neurovirol. 2019;25(3): 313-323.
18. Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010:140(6): 918-934.
19. Alshelh Z, Mills EP, Kosanovic D, et al. Effects of the
glial modulator palmitoylethanolamide on chronic pain intensity and brain
function. Journal of pain research; 2019; 12, 2427-2439.
20. Ruili Li, Wei Wang, Yuanyuan Wang, et al. Effects of early HIV infection and combination antiretroviral therapy on intrinsic brain activity: a cross-sectional resting-state fMRI study. Neuropsychiatr Dis Treat. 2019;15: 883–894.
21. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23): 2087–2096.
Figures