4655
Sex Differences across Brain Regions in Neonates1BioMedIA, Department of Computing, Imperial College London, London, United Kingdom, 2Centre for the Developing Brain, Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Sir Peter Mansfield Imaging Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom
Synopsis
We present for the first time a large cohort of neonatal brain MR images were acquired. Based on 505 subjects' MRI brain imaging data were acquired as part of the developing Human Connectome Project (dHCP) , we investigated the differences of 92 brain regions and brain volume without and with normalization of the volumes. Using multivariate analysis and adaptive step-down false discovery rate (FDR) control methods to examine term born neonates, we found 90 out of 92 regions and the brain volume statistically significant between sexes. But selected data normalization methods also suggested less statistical significance in neonatal brain regions.
Purpose
Studies on difference of brain regions across sex are not only biological interest and importance, but also psychiatrically and pathologically meaningful to understand brain-based diseases, such as autism spectrum disorders (ASD), Alzheimer’s, and social communication disorder (SCD) [1-3]. Therefore it is important to understand the difference in brain regions associated with sex. Previous studies have focused either on young children and adults. However, there is a lack of studies during early brain development. In this abstract we investigate differences in regional brain volumes in neonates.Methods
Using the 505 neonatal T2 weighted MRI brain images acquired in the developing Human Connectome Project (dHCP) [4] at St. Thomas Hospital (London) on a 3T Philips Achieva scanner using a specially designed 32 channel neonatal head coil [5]. Infants were imaged without sedation except for those who were indicated. The ages of the neonates range from 29.3 weeks to 45.1 weeks. The dataset included 222 female and 283 male neonates, including 192 term born females and 226 term born males. The age distribution of neonates at scan time is shown in Figure 1. For each subject, the T2 brain MR image was segmented into 92 regions, including cerebrospinal fluid (CSF), cortical gray matter (CGM), white matter (WM), ventricles, cerebellum, brainstem and other 86 regions, using the structural image analysis pipeline [6]. Because lacking of knowledge about healthiness of preterm born babies, we excluded the preterm born subjects. We also excluded 12 term born females and 11 term born males due to missing body weight data. Using multivariate analysis method and p-value adjustment by adaptive step-down false discovery rate (FDR) control [7], 92 brain regions and brain volume were inspected across term birth ages, that is > 37 weeks, by examining the absolute volumes, volumes normalized to brain volume, and volumes normalized to body weight at scan.Results
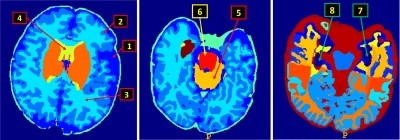
Among the 92 brain regions and the brain volume, with absolute volumes, there were 81 statistically significant regions. After applying adaptive step-down FDR control for p-value correction, 90 regions showed statistical significance (p-value < 0.05). Different results were observed when regional data were normalized to brain volume and body weight respectively. After regional volumes normalized to brain volume, there were 29 regions showed statistical significance (p-values unadjusted) and 23 regions had statistical significance (p-values adjusted). While normalization to body weight suggested only 5 statistically significant regions (p-values unadjusted) and no statistical significance (p-values adjusted) in all regions. Due to limited space, only 8 regions are shown in Figure 2 and Table 1.Conclusion
Existing studies based on adults and young children have revealed some differences among brain regions between females and males. However, the differences in brain regions at early ages remain unknown. By examining the first large cohort of neonatal brain imaging data, we had a preliminary study on the difference of neonatal brain regions for the first time. This abstract provided an overview on the differences in neonatal brain regions under certain normalization methods. We hope this abstract will be able to deliver essential links to future studies regarding brain-based diseases in youth and adults.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union Seventh Framework Programme (FP/20072013)/ERC Grant Agreement no. 319456. We are grateful to the families who generously supported this trial. The work was supported by the NIHR Biomedical Research Centres at Guys and St Thomas NHS Trust.References
1. Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, Liewald DCM, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cerebral Cortex 2018;28(8):2959-2975.
2. Mazure CM, Swendsen J. Sex differences in Alzheimer's disease and other dementias. Lancet Neurol 2016;15(5):451-452.
3. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007;62(8):847-855.
4. Makropoulos A, Robinson EC, Schuh A, Wright R, Fitzgibbon S, Bozek J, Counsell SJ, Steinweg J, Vecchiato K, Passerat-Palmbach J, Lenz G, Mortari F, Tenev T, Duff EP, Bastiani M, Cordero-Grande L, Hughes E, Tusor N, Tournier J-D, Hutter J, Price AN, Teixeira RPAG, Murgasova M, Victor S, Kelly C, Rutherford MA, Smith SM, Edwards AD, Hajnal JV, Jenkinson M, Rueckert D. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. NeuroImage 2018;173:88-112.
5. Hughes EJ, Winchman T, Padormo F, Teixeira R, Wurie J, Sharma M, Fox M, Hutter J, Cordero-Grande L, Price AN, Allsop J, Bueno-Conde J, Tusor N, Arichi T, Edwards AD, Rutherford MA, Counsell SJ, Hajnal JV. A dedicated neonatal brain imaging system. Magn Reson Med 2017;78(2):794-804.
6. Makropoulos A, Gousias IS, Ledig C, Aljabar P, Serag A, Hajnal JV, Edwards AD, Counsell SJ, Rueckert D. Automatic Whole Brain MRI Segmentation of the Developing Neonatal Brain. IEEE Transactions on Medical Imaging 2014;33(9):1818-1831.
7. Gavrilov Y, Benjamini Y, Sarkar SK. An adaptive step-down procedure with proven FDR control under independence. Ann Statist 2009;37(2):619-629.