4643
Greater reduction in functional connectivity strength in term neonates with congenital heart disease born earlier in gestation1Radiology, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 2University of Pittsburgh, Pittsburgh, PA, United States, 3Children's Hospital of Los Angeles, Los Angeles, CA, United States
Synopsis
Functional connectivity strength (FCS) differences are investigated in term neonates with congenital heart disease (CHD) as compared to normal controls. In CHD neonates, FCS is reduced in default mode network (DMN), salience network (SN), and central executive network (CEN) regions. However, widespread positive post-conceptional age (PCA) at birth – X – CHD interactions were found, indicating this effect is more pronounced for CHD neonates born earlier in gestation, and that prolonged in utero exposure may be beneficial for development of brain functional connectivity. Negative correlations of nasal nitric oxide (nNO) and FCS were also found, suggesting a partly vascular etiology.
Introduction
Congenital heart disease (CHD) is associated with brain dysmaturation and functional network connectivity differences from infancy onwards1,2 and carries greater risk of adverse neurocognitive outcome3. However, the possible effect of post-conceptional age (PCA) at birth in infants normally labeled “term” (>= 37 weeks) has not been investigated in detail. Here we investigate differences in functional connectivity strength (FCS) and regional cerebral blood flow (CBF) between CHD neonates and normal controls and interactions with PCA at birth, as well as correlations with nasal nitric oxide (nNO).Materials and Methods
Data was acquired at Children’s Hospital of Pittsburgh of UPMC (CHP) on a 3T Siemens Skyra scanner and a 3T GE Excite scanner, and at Children’s Hospital Los Angeles (CHLA) on a 3 T Philips Achieva scanner. CHD infants were heterogenous with regard to type of heart lesion with no frank visible injury.Resting-state BOLD data was successfully acquired from 218 term (>= 37 weeks PCA at birth) infants (89 CHD, 129 Control) under normal sleep without sedation using a feed-and-bundle technique. Demographic information is given in Figure 1. Previously published methods were used to minimize the risk of spurious correlations arising from participant motion4. Functional connectivity strength (FCS) maps were generated as follows. A neonatal specific parcellation atlas5 was used to determine gray matter voxels. The FCS for a gray matter voxel is defined as the mean correlation coefficient between that voxel and all other gray matter voxels after regressing out motion correction and drift parameters and band-pass filtering; negative values of correlation coefficient were set to zero. PCASL imaging was successfully performed in a smaller subset of subjects (N = 35) at CHP. CBF maps were computed after motion correction using the two-compartment model6 and normalized into standardized space.
A voxelwise GLM was used with CHD status the independent variable; PCA at scan date, PCA at birth, scanner, voxel size, multiband parameter as covariates of no interest; and FCS or CBF as dependent variable. Results were Gaussian filtered with σ = 4 mm and standard methods7 were used to correct for multiple comparisons across voxels. The analysis was repeated investigating PCA at birth – X – CHD interactions on FCS. Results were deemed significant at p < 0.05 family-wise error (FWE) corrected.
For a subset (N = 27) of the study population, nNO levels were available using tidal breath sampling from each naris using a CLD 88sp NO analyzer. nNO was correlated with FCS on a voxelwise basis using the above procedure. nNO is a proxy for cerebral NO bioavailability as endothelial NO synthase (eNOS) is present both in the nasal cavity and in the brain.
Results
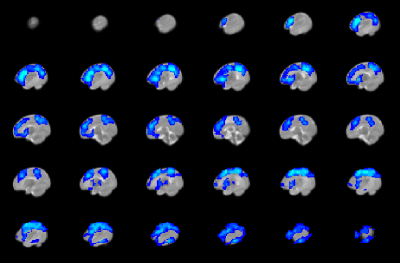
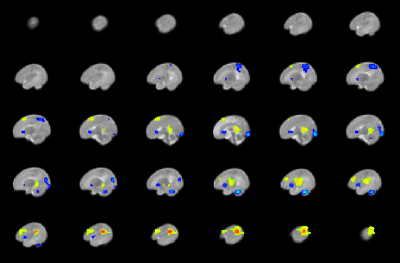
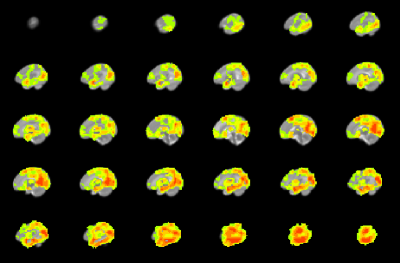
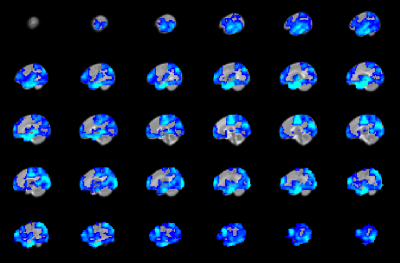
CHD neonates display lower FCS (Figure 2) mainly in medial prefrontal, dorsolateral prefrontal, precuneus/posterior cingulate, anterior cingulate, and frontoinsular regions, which primarily make up the default mode network (DMN), salience network (SN), and central executive network (CEN). In contrast, CHD neonates display altered CBF in subcortical and primary sensi-motor and visual regions (Figure 3). Widespread regions show CHD-X-PCA at birth interactions on FCS (Figure 4), including the regions with lower FCS in CHD neonates and concentrated in posterior and frontoinsular regions. nNO is also negatively correlated with FCS in these regions (Figure 5).Discussion
Agreeing with a previous report2, results demonstrate that brain dysmaturation in CHD neonates includes differences in functional connectivity. FCS maps provide an alternative to other analysis strategies such as graph analysis and investigate connectivity at a finer scale compared to topology. Here reduced FCS is seen in three networks critical for cognitive function (DMN, SN, CEN) and may underlay later adverse neurocognitive outcome in CHD patients. Future research associating connectivity in infancy with later outcome will be necessary to investigate this hypothesis further.However, the CHD-X-PCA at birth interactions indicate that this effect is significantly more pronounced for CHD infants born earlier in gestation, even when such birth is conventionally at term (>= 37 weeks). Prolonged in utero exposure may therefore have a beneficial effect on brain development in CHD infants, although similar genetic effects may delay PCA at birth as well as foster development of functional connectivity. Future research will investigate whether a similar effect is present in CHD infants born preterm.
The negative correlation seen between nNO and FCS suggests these results may be partly vascular in origin. NO is a neurotransmitter which mediates cerebral autoregulation and neuronal-vascular coupling via receptors in the blood vessels8. Impaired ability for NO reception would result in increased NO levels but also decreased functional connectivity as the magnitude of the BOLD effect will be reduced. The abnormalities in FCS and CBF were co-localized to separate regions (FCS to multi-modal regions, CBF to subcortical and primary modal regions), suggesting that CHD disrupts neuronal-metabolic-vascular interactions.
Conclusion
FCS is reduced in term CHD neonates in DMN, CEN, and SN regions compared to controls. This effect is significantly larger in CHD neonates with lower PCA at birth and is likely vascular in origin as evidenced by correlations with nNO. Results indicate prolonged in utero exposure may have a beneficial effect on development of functional connectivity in CHD infants.Acknowledgements
No acknowledgement found.References
1. Leonetti C, Back SA Gallo V, Ishibashi N. Cortical Dysmaturation in Congenital Heart Disease. Trends Neurosci 2019; 42(3): 192-204.
2. De Asis-Cruz J, Donofrio MT, Vezina G, Limperopoulos C. Aberrant brain functional connectivity in newborns with congenital heart disease before cardiac surgery. Neuroimage Clin 2018; 17: 31-42.
3. Marelli A, Miller SP, Marino BS, Jefferson AL, Newburger JW. The Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation 2016; 133(20): 1951-1962.
4. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fmri. NeuroImage. 2014; 84: 320-341.
5. Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D. Infant Brain Atlases from Neonates to 1- and 2-Year Olds. PLoS ONE 2011; 6(4): e18746.
6. Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 tesla. Magnetic resonance in medicine. 2002;48:242-254
7. Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3d clusters in functional brain images. NeuroImage. 1998;8:113-128
8. Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: Chemoregulation versus mechanoregulation. Circulation. 2003;107:1901-1905
Figures