4642
Linked signatures of brain structure and microstructure at preschool age predict reading readiness in early childhood1Radiology, University of Calgary, Calgary, AB, Canada, 2Owerko Centre, Alberta Children Hospital Research Institute, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 4Pediatrics, University of Calgary, Calgary, AB, Canada, 5Community Health Sciences, University of Calgary, Calgary, AB, Canada
Synopsis
We aimed to identify whether variations in grey and white matter brain structure during early childhood predict future pre-reading skills. We examined anatomical (T1-weighted) and diffusion tensor (DTI) images from 35 children at 3.5years(±3months). Children were assessed for their pre-reading abilities using NEPSY-II subtests one year later (4.5years±3months). A data-driven linked independent component analysis was used to identify components of DTI and morphometry measures with shared variability across subjects that related to pre-reading ability approximately a year later. Our results suggest the co-development of grey and white matter brain structures in early life predicts future pre-reading capabilities in preschool children.
Introduction
Language development is a dynamic process that occurs throughout childhood and lays the foundation for future reading skills, which play a fundamental role in academic achievement, social engagement, and mental health.1-4 The specific neurobiological processes underlying the development of reading skills take place very early in life, and recent research suggests that variation in brain structure during early childhood precedes reading difficulties.5-8 We aimed to identify variations in brain structure during early childhood that predict future pre-reading skills. A better understanding of how the development of brain structures prior to reading instruction may influence future pre-reading and reading skills is essential in order to identify an optimal window for implementation of successful early interventions to improve later reading skills, and may help identify key regions to target for early intervention approaches.Methods
This study includes 35 children aged 3.25-3.75 years (19 boys) selected from a larger ongoing study (Calgary Preschool MRI Study).9 Inclusion criteria were successful T1-weighted and DTI scans at 3.5 years ± 3 months, as well as a follow up pre-reading assessment using the NEPSY-II Phonological Processing and Speeded Naming subtests10 at 4.5 years ± 3months.Imaging was conducted using a GE 3T MR750w scanner and 32-channel head coil. Whole-brain DTI was acquired using a single shot spin echo echo-planar imaging sequence, with TE=79ms, TR=6750ms; 30 gradient directions at b=750 s/mm2, and 5 volumes at b=0 s/mm2. T1-weighted images were acquired using a FSPGR BRAVO sequence, 210 axial slices; 0.9 x 0.9 x 0.9mm resolution, TE = 3.76 ms, TR = 8.23 ms, flip angle = 12º, matrix size = 512 x 512, inversion time = 540 ms.
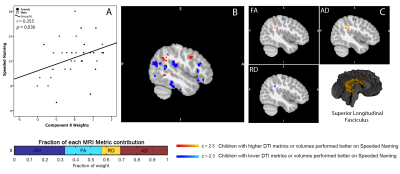
Standard VBM processing was undertaken using FSL,11 with the NIHPD asymmetrical pediatric brain template (ages 4.5-8.5 years) in Montreal Neurological Institute (MNI) standard space.12 DTI processing was undertaken using a standard processing pipeline in ExploreDTI, following which fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) maps were extracted and non-linearly registered to the same pediatric template. Tract-based spatial statistics (TBSS) was run using FSL, and masked using the JHU atlas,11,13-15 to select reading-related tracts (bilateral inferior fronto-occipital/inferior longitudinal, superior longitudinal (SLF) and uncinate fasciculi). The VBM and masked TBSS images were then used as input for a linked independent component analysis using FSL tools. This is a data-driven approach that identifies components of multiple modalities or imaging parameters (e.g., DTI and morphometry measures) that share variability across subjects (e.g., biological variations).16
Partial correlations, controlling for sex, were run in SPSS to determine the relationship between component weights (linked ICA output) and pre-reading measures.
Results
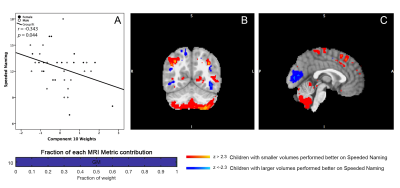
Two linked components significantly correlated with pre-reading measures. One component (Component 8) involved volumetric variability in the lingual gyrus, thalamus, fusiform gyrus, middle temporal regions, and the dorsolateral prefrontal cortex, linked with bilateral changes in FA and AD along the superior longitudinal fasciculus. Component 8 was significantly correlated with Speeded Naming (r=0.355, p=0.036) one year later (Figure 1). Of note, larger volumes in the thalamus, fusiform gyrus, and middle temporal area, smaller volumes in the supramarginal and angular gyri and the dorsolateral prefrontal cortex, and higher FA and AD/lower RD in the bilateral SLF at 3.5 years were correlated with better pre-reading at 4.5 years.A second component (Component 10) was dominated by volumetric changes in the cerebellum, precuneus, angular gyri and areas in the occipital cortex (visual regions), and significantly correlated with Speeded Naming (r=-0.343, p=0.044) one year later (Figure 2). Smaller volumes in the cerebellum, and supramarginal and angular gyri, and larger volumes in visual areas in the occipital lobe at 3.5 years were correlated with better pre-reading at 4.5 years.
Discussion and Conclusion
Our study identified variations in brain structure prior to formal reading instruction that predict future pre-reading skills during early childhood. This is consistent with recent findings that microstructure of the arcuate fasciculus during infancy is associated with language skills in kindergarten.8 Contributions from both cortical grey matter volume and white matter microstructure suggest a shared development through refinement and growth that underlie the development of pre-reading during early childhood. Importantly, shared variation in reading related grey matter structures (e.g. supramarginal and angular gyrus, and dorsolateral prefrontal cortex) are structurally connected via the superior longitudinal SLF, where variation in white matter integrity was observed (Component 8). The linked reading-related components represent the complex development of local white matter microstructure that directly connect reading-related areas of the cortex as well as refinement through integration of whole-brain network architecture. Variation in reading related grey matter regions including the cerebellum, and visual areas was also related to later pre-reading skill level (Component 10). This may suggest an earlier refinement of visual reading related regions in children who have better pre-reading skills. Together these results suggest that the co-development of both white and grey matter brain structures in early life predict future pre-reading capabilities in preschool children.Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR) (funding reference numbers IHD-134090, MOP-136797, New Investigator Award to C.L). J.E.R was supported by an Eyes High University of Calgary Postdoctoral Scholarship, the T. Chen Fong Postdoctoral Fellowship in Medical Imaging Science, and a CIHR Postdoctoral Fellowship (MFE-164703). K.Y.M was supported by an NSERC Postdoctoral Fellowship. D.P. was supported by an Owerko Postdoctoral Scholarship. The authors thank members of the APrON study for assistance with recruitment.References
1. Dehaene-Lambertz G, Hertz-Pannier L, Dubois, J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci. 2006;29:367-373.
2. Lonigan CJ, Farver JM, Nakamoto J, Eppe S. Developmental trajectories of preschool early literacy skills: a comparison of language-minority and monolingual-English children. Dev Psychol. 2013;49:1943-1957.
3. May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front Psychol. 2011;2:222.
4. Torppa M, Poikkeus A-M, Laakso M-L, Eklund K, Lyytinen H. Predicting delayed letter knowledge development and its relation to grade 1 reading achievement among children with and without familial risk for dyslexia. Dev Psychol. 2006;42:1128-1142.
5. Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage. 2011;57:742-749.
6. Reynolds JE, Long X, Grohs MN, Dewey D, Lebel C. Structural and functional asymmetry of the language network emerge in early childhood. Dev Cog Neurosci. 2019;39:100682.
7. Walton M, Dewey D, Lebel C. Brain white matter structure and language ability in preschool-aged children. Brain Lang. 2018;176:19-25.
8. Zuk J, Sanfilippo J, Figuccio MJ, Dunstan J, Carruthers C, Sideridis G, Gagoski B, Grant PE, Gaab N. White matter in infancy is prospectively associated with language outcome in kindergarten. bioRxiv. 2019; https://doi.org/10.1101/781914
9. Reynolds JE, Long X, Paniukov D, Lebel C. Calgary Preschool MRI Dataset. Open Science Forum; 2019. DOI 10.17605/OSF.IO/AXZ5R
10. Korkman M, Kirk U, Kemp S. NEPSY–Second Edition (NEPSY-II). San Antonio: The Psychological Corporation; 2007.
11. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782-790.
12. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313-327.
13. Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39:336-347.
14. Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher L. MRI atlas of human white matter. Elsevier; 2005.
15. Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S.Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630-644.
16. Groves AR, Beckmann CF, Smith SM, Woolrich MW. Linked independent component analysis for multimodal data fusion. NeuroImage. 2011;54:2198-2217.
Figures