4641
Alterations in brain functional connectivity in pediatric migraine1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Hotchkiss Brain Institute, Calgary, AB, Canada, 3Alberta Children's Hospital Research Institute, Calgary, AB, Canada, 4Department of Psychology, University of Calgary, Calgary, AB, Canada, 5Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
Synopsis
Though migraine is a common childhood disease, there has been relatively little investigation into migraine in children. Here we use resting-state functional connectivity analyses to investigate functional alterations in brain activity in children with migraine. In agreement with the adult literature, we show altered resting-state connectivity in children with migraine compared to healthy controls in areas related to sensory processing.
INTRODUCTION
Migraine is a chronic, painful, debilitating condition characterised by recurrent, severe headache. Migraine is one of the top five most frequent childhood diseases, affecting over 10% of children aged 5-151. Early intervention can decrease migraine frequency, with those receiving earlier interventions more likely to achieve remission. However, treatment strategies for children are limited, in part due to a lack of knowledge about migraine biology, and a reluctance to prescribe medications due to undesirable side effects and limited efficacy2. To develop more effective treatments, it is important to better understand the mechanisms behind migraine in children.Though the most well-known symptom, headache only reflects a specific phase of migraine. During the periods between headaches, though generally asymptomatic, adults and adolescents with migraine show altered brain functional connectivity. In adults with migraine, connectivity has been shown to be altered in the pain processing network, affective network, default mode network, executive control network, salience network and visual network.3 In adolescents, there is altered connectivity in the auditory, control, default mode, salience, fronto-parietal and sensorimotor networks.4 Despite the strong evidence for altered interictal connectivity in migraine, there has been little attempt to investigate this in children. The aim of this study was to investigate resting-state functional connectivity differences between children age 7-13 with migraine and healthy, age matched controls.
METHODS
Scanning was performed on a 3T GE 750w MR scanner using a 32-channel head coil. A T1-weighted anatomical image was collected for registration (BRAVO; TE/TR = 2.7/7.4ms, slice = 1 mm3 isotropic voxels). Resting state data was collected using a T2*-weighted echo planar imaging sequence (165 volumes, TE/TR = 29/2500 ms, slice thickness = 3.5mm, matrix size = 64 x 64). During the sequence subjects watched the movie INSCAPES.5Analyses were performed using FSL.6 Data were preprocessed using the following steps: brain extraction (BET),7 motion correction (MCFLIRT),8 slice timing correction, spatial smoothing (6.0 mm) high pass filtering (100 s). Subjects were excluded if peak motion was higher than 5 mm. Single subject ICA was run in MELODIC and noise components were removed using the criteria specified in Ref 9. Group ICA and dual regression were used to compare 30 resting-state network components across groups using threshold-free cluster enhancement.
RESULTS
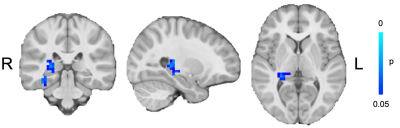
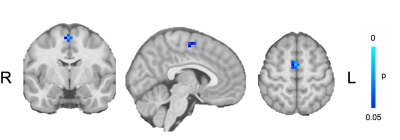
Children with migraine had lower connectivity between the hippocampus and thalamus with the sensorimotor network (Figure 1) and higher connectivity between the supplementary motor area and the salience network (Figure 2).DISCUSSION
Resting-state functional connectivity analysis showed decreased connectivity between the hippocampus and thalamus with the sensorimotor network in children with migraine compared to controls and higher connectivity between the supplementary motor area and the salience network.It is hypothesised that migraine is a consequence of a hyperexcited cortex, resulting from abnormal thalamic control. Thalamocortical activity has been shown to be reduced between migraine attacks in adults and relates to features such as attack severity and duration.10 The reduced connectivity between the thalamus and sensorimotor network shown here in children with migraine supports this theory. Additionally, the sensorimotor areas provide top-down regulation to the trigemino-thalamo-cortical nociceptive pathway, known to play a key role in migraine. Dysfunction in this network affects the perception and processing of pain.3
The salience network integrates sensory, emotional and cognitive inputs and is often considered a filtration/amplification network. Higher headache frequency and pain sensitivity has been shown to be associated with reduced salience network connectivity overall in adults,11 however adults with migraine have shown increased connectivity between the salience network and sensory areas such as the visual cortex,12 complementing our finding of increased connectivity between the salience network and the supplementary motor area. This increased connectivity with sensory areas may reflect a hypervigilance to sensory stimuli in children with migraine.
CONCLUSION
We show alterations in connectivity in sensory areas in children with migraine compared controls suggesting that migraine is a disorder of sensory processing and the sensory relevance of stimuli. These network alterations are seen between migraines, providing insight to the baseline differences in sensory information processing in pediatric migraine.Acknowledgements
This project was funded by the SickKids Foundation and CIHR Institute of Human Development, Child and Youth Health New Investigator award. TB is supported by the Harley N. Hotchkiss Postdoctoral Fellowship.References
1. Maleki N, Bernstein C, Napadow V, Field A. Migraine and Puberty: Potential Susceptible Brain Sites. Semin Pediatr Neurol. 2016;23(1):53-59. doi:10.1016/j.spen.2016.01.011
2. Powers SW, Coffey CS, Chamberlin LA, et al. Trial of Amitriptyline, Topiramate, and Placebo for Pediatric Migraine. N Engl J Med. 2017;376(2):115-124. doi:10.1056/NEJMoa1610384
3. Zhang J, Su J, Wang M, et al. The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol. 2017;264(4):654-663. doi:10.1007/s00415-017-8404-4
4. Colon E, Ludwick A, Wilcox SL, et al. Migraine in the Young Brain: Adolescents vs. Young Adults. Front Hum Neurosci. 2019;13(March):1-25. doi:10.3389/fnhum.2019.00087
5. Vanderwal T, Kelly C, Eilbott J, Mayes L, Castellanos FX. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;15(122):222-232. doi:doi:10.1016/j.neuroimage.2015.07.069
6. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208-219.
7. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-155.
8. Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved Optimisation for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage. 2002;17(2):825-841.
9. Griffanti L, Douaud G, Bijsterbosch J, et al. Hand classification of fMRI ICA noise components. Neuroimage. 2017;154(June 2016):188-205. doi:10.1016/j.neuroimage.2016.12.036
10. Coppola G, Bracaglia M, Di Lenola D, et al. Lateral inhibition in the somatosensory cortex during and between migraine without aura attacks: Correlations with thalamocortical activity and clinical features. Cephalalgia. 2015;36(6):568-578. doi:10.1177/0333102415610873
11. Androulakis XM, Krebs K, Peterlin BL, et al. Modulation of intrinsic resting-state fMRI networks in women with chronic migraine. Neurology. 2017;89(2):163-169. doi:10.1212/WNL.0000000000004089
12. Hubbard CS, Khan S a, Keaser ML, Mathur V a, Goyal M, Seminowicz D a. Altered Brain Structure and Function Correlate with Disease Severity and Pain Catastrophizing in Migraine Patients. eNeuro. 2014;1(1):e20.14. doi:10.1523/ENEURO.0006-14.2014
Figures